
6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine synthesis
- Product Name:6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine
- CAS Number:570415-48-4
- Molecular formula:C14H15ClN4
- Molecular Weight:274.75
![2-AMINO-5,6,7,8-TETRAHYDRO-6-(PHENYLMETHYL)PYRIDO[4,3-D]PYRIMIDIN-4(3H)-ONE](/CAS/GIF/1029-52-3.gif)
1029-52-3
11 suppliers
$150.00/0.5g
![6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine](/CAS/20130318/GIF/CB32645431.gif)
570415-48-4
7 suppliers
inquiry
Yield:570415-48-4 57%
Reaction Conditions:
Stage #1: 2-amino-6-benzyl-5,6,7,8-tetrahydropyrido<4,3-d>pyrimidin-4(3H)-onewith trichlorophosphate in N,N-dimethyl-formamide at 110;Inert atmosphere;
Stage #2: with ammonia in N,N-dimethyl-formamide; pH=4;Cooling with ice;
Steps:
Synthesis of 6-benzyl-4-chloro-5,6,7,8-tetrahydro-pyrido[4,3-d] pyrimidin-2-ylamine: Pentanoic acid [6-benzyl-4-(2-chloro-phenyl)-5,6,7,8-tetrahydro-pyrido[4,3- d]pyrimidin-2-yl]-amide (4.4g,. 17.2mmoles) was added portionwise to stirred phosphorus oxychloride (40mIs). One drop of DMF was added to the reaction mixture and the yellow suspension was stirred and heated (1100C) under nitrogen gas for 3.5 hours to generate a brown/yellow solution.After cooling to room temperature the reaction mixture was poured onto ice/water (500mIs) with rapid stirring to generate an orange/yellow solution. The solution was cooled in ice and the pH adjusted to pH4 with concentrated ammonia. The solution was left overnight to produce a cloudy solution with pH9.0 and the solid was collected, washed with water (100mls) and dried in a vacuum desiccator to yield an orange/yellow solid (2.7g,. 57% yield). LC-MS (retention 2.50mins., mass 275 M+H).
References:
WO2010/125343,2010,A1 Location in patent:Page/Page column 29; 30
![2-AMINO-5,6,7,8-TETRAHYDRO-6-(PHENYLMETHYL)PYRIDO[4,3-D]PYRIMIDIN-4(3H)-ONE](/CAS/GIF/1029-52-3.gif)
1029-52-3
11 suppliers
$150.00/0.5g
![6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine](/CAS/20130318/GIF/CB32645431.gif)
570415-48-4
7 suppliers
inquiry
![2-AMino-7-benzyl-5,6,7,8-tetrahydro-3H-pyrido[3,4-d]pyriMidin-4-one](/CAS/20150408/GIF/62458-92-8.gif)
62458-92-8
13 suppliers
$200.00/1g
![6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine](/CAS/20130318/GIF/CB32645431.gif)
570415-48-4
7 suppliers
inquiry
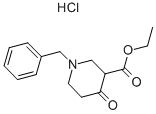
1454-53-1
233 suppliers
$5.00/5g
![6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2-aMine](/CAS/20130318/GIF/CB32645431.gif)
570415-48-4
7 suppliers
inquiry