
1,2,3,4-TETRAHYDRO-ISOQUINOLINE-3-CARBOXYLIC ACIDETHYL ESTER HYDROCHLORIDE synthesis
- Product Name:1,2,3,4-TETRAHYDRO-ISOQUINOLINE-3-CARBOXYLIC ACIDETHYL ESTER HYDROCHLORIDE
- CAS Number:57980-74-2
- Molecular formula:C12H16ClNO2
- Molecular Weight:241.72
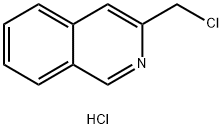
76884-33-8
24 suppliers
$177.00/100mg
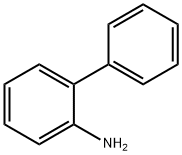
90-41-5
343 suppliers
$16.00/5g
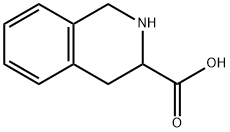
67123-97-1
130 suppliers
$9.00/1g

57980-74-2
20 suppliers
$82.50/1g
Yield:-
Reaction Conditions:
with hydrogenchloride;formaldehyd;potassium carbonate in water
Steps:
g Notes
g. Three equivalents of potassium carbonate were used. 3-Chloromethylisoquinoline hydrochloride, used as a starting material, was obtained as follows: A mixture of phenylaniline (40 g), formaldehyde (37% w/v in water, 91 ml) and concentrated hydrochloric acid (310 ml) was stirred and heated to reflux for 4 hours and then stored at ambient temperature for 16 hours. The precipitate was filtered off, washed with cold water and with acetone to give 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (11 g). After appropriate repetition of the above step a mixture of the product so obtained (23.2 g) and methanol (200 ml) was cooled in an ice-bath and thionyl chloride (15.4 ml) was added dropwise. The mixture was heated to reflux for 4 hours. The mixture was evaporated and the solid residue was triturated in diethyl ether to give ethyl 1,2,3,4-tetrahydroisoquinoline-3-carboxylate hydrochloride (23.2 g). NMR Spectrum: (CD3 SOCD3, delta values) 3.1-3.4(m, 2H), 3.8(s, 3H), 4.32(s, 2H), 4.5-4.6(q, 1H), 7.3(s, 4H), 10.2(broad s, 1H).
References:
Imperial Chemical Industries PLC;ICI Pharma US5134148, 1992, A