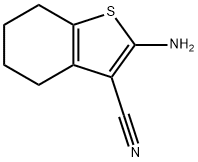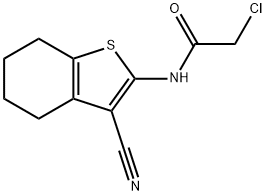
2-CHLORO-N-(3-CYANO-4,5,6,7-TETRAHYDRO-1-BENZOTHIOPHEN-2-YL)ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(3-CYANO-4,5,6,7-TETRAHYDRO-1-BENZOTHIOPHEN-2-YL)ACETAMIDE
- CAS Number:58125-40-9
- Molecular formula:C11H11ClN2OS
- Molecular Weight:254.74
Yield:58125-40-9 91%
Reaction Conditions:
in 1,4-dioxane at 20; for 15 - 65 h;
Steps:
2
To a stirred solution of the appropriate aminothiophene (1 equiv. ) in 1,4-dioxane (5ml per mmol aminothiophene) was added portionwise the appropriate acyl chloride (1.15 equiv. ). The reaction mixture was stirred at room temperature for 17-65 hours. The solvent was removed under reduced pressure, the residue triturated with hexane, a hexane/ether mixture or ether, and filtered to give the desired product. Compound No. 69 of Table 14 Using the alternative general procedure 2-chloro-N- (3-cyano-4, 5,6, 7-tetrahydro- benzo [b] thiophen-2-yl) -acetamide (Compound No. 69 of Table 14) was obtained from aminothiophene EO as a pale pink solid in 91% yield. 1H NMR on (400MHz, CDC13) : 9.25 (1H, br s), 4.25 (2H, s), 2.60 (4H, m) and 1.80 (4H, m) ppm.
References:
WO2005/44008,2005,A2 Location in patent:Page/Page column 113

108-94-1
527 suppliers
$12.00/50g

58125-40-9
24 suppliers
inquiry