5-AMINO-1-(4-CHLOROPHENYL)-3-METHYL-1H-PYRAZOLE-4-CARBONITRILE synthesis
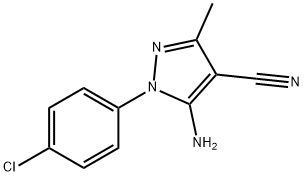
- Product Name:5-AMINO-1-(4-CHLOROPHENYL)-3-METHYL-1H-PYRAZOLE-4-CARBONITRILE
- CAS Number:58791-82-5
- Molecular formula:C11H9ClN4
- Molecular Weight:232.67
Yield: 86%
Reaction Conditions:
with sodium acetate in ethanol for 1 h;Reflux;
Steps:
General Procedure for the synthesis of compounds 5-amino-1-aryl-3-methyl-1H-pyrazole-4-carbonitrile 2a-m.
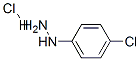
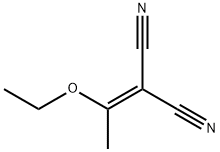
General procedure: Arylhydrazine hydrochlorides (0.01mol) were reacted with (1-ethoxyethylidene)malononitrile (0.01 mol) and sodium acetate (0.02mol) in ethanol (40mL), under reflux, during 1h. After, the mixture was poured in cold water and the precipitate formed was filtered out and recrystallized from ethanol/water. The reactions were accomplished by means of TLC using silica gel plate with fluorescent indicator and hexane/ethyl acetate(1:1) as eluent.
References:
Faria, Jéssica V.;Dos Santos, Maurício S.;Bernardino, Alice M.R.;Becker, Klaus M.;Machado, Gérzia M.C.;Rodrigues, Raquel F.;Canto-Cavalheiro, Marilene M.;Leon, Leonor L. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 23,p. 6310 - 6312] Location in patent:supporting information