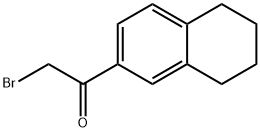
2-bromo-1-(5,6,7,8-tetrahydronaphthalen-2-yl)ethanone synthesis
- Product Name:2-bromo-1-(5,6,7,8-tetrahydronaphthalen-2-yl)ethanone
- CAS Number:5896-66-2
- Molecular formula:C12H13BrO
- Molecular Weight:253.13
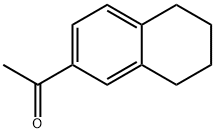
774-55-0
87 suppliers
$44.00/5g

5896-66-2
9 suppliers
$203.00/250mg
Yield:5896-66-2 89%
Reaction Conditions:
with bromine in methanol at 0 - 20; for 18.75 h;
Steps:
4.A
Example 4 - Preparation of 2-chloro-6-(5',6',78Metrahvdronaphthalen-2'- vnimidazoβ.l-blthiazole f Compound 25); A. Preparation of 2-bromo-l-(5',6',7',8'-tetrahydronaphthalen-2'- yl)ethanone; Bromine (0.63 mL, 12.3 mmol) was added to a stirred solution of l-(5',6',7',8'- tetrahydronaphthalen-2'-yl)ethanone (2.13 g, 12.3 mmol) in methanol (15 mL) at O0C and the resultant solution allowed to stir for 2.75 hours before the reaction mixture was diluted with water and allowed to warm to room temperature over 16 hours. After this time, the reaction mixture was extracted with ethyl acetate:40-60 petrol
References:
WO2009/13477,2009,A1 Location in patent:Page/Page column 23-24
119-64-2
341 suppliers
$9.00/25g
598-21-0
311 suppliers
$10.00/10g

5896-66-2
9 suppliers
$203.00/250mg

119-64-2
341 suppliers
$9.00/25g

5896-66-2
9 suppliers
$203.00/250mg