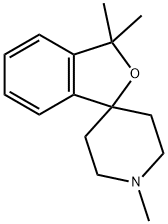
1,3,3-trimethyl-3H-spiro[isobenzofuran-1,4-piperidine] synthesis
- Product Name:1,3,3-trimethyl-3H-spiro[isobenzofuran-1,4-piperidine]
- CAS Number:59043-56-0
- Molecular formula:C15H21NO
- Molecular Weight:231.33
![4-Piperidinol, 4-[2-(1-hydroxy-1-methylethyl)phenyl]-1-methyl-](/CAS/20210305/GIF/59043-46-8.gif)
59043-46-8
0 suppliers
inquiry
![1,3,3-trimethyl-3H-spiro[isobenzofuran-1,4-piperidine]](/CAS/20180703/GIF/59043-56-0.gif)
59043-56-0
7 suppliers
inquiry
Yield:59043-56-0 56%
Reaction Conditions:
Stage #1: 4-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-1-methyl-piperidin-4-olwith boron trifluoride diethyl etherate in benzene at 20; for 40 h;
Stage #2: with sodium hydroxide in water;benzene;
Steps:
66.3
To a stirred solution of 4-[2-(1-hydroxy-1-methylethyl) phenyl]-1- methylpiperidin-4-ol (step 2,4. 62 g, 18.5 mmol) in benzene (200 mL) was added dropwise boron trifluoride diethyl etherate (11.0 mL, 86.8 mmol) at room temperature and the mixture was stirred for 40 h at the same temperature. The reaction mixture was quenched by the addition of water (200 mL) and 2 N sodium hydroxide aqueous solution (200 mL), and the benzene layer was separated. The aqueous layer was extracted with diethyl ether, and then combined organic layer was washed with brine, dried over sodium sulfate, and evaporated. The residue was purified by column chromatography on an amine coated silica gel (100 g) eluting with dichloromethane to afford 2.39 g (56%) of the title compound as a colorless solid: 'H-NMR (CDCl3) 8 7.30-7. 24 (2H, m), 7.12-7. 07 (2H, m), 2.81-2. 72 (2H, m), 2. 51- 2.42 (2H, m), 2.37 (3H, s), 2.07-1. 97 (2H, m), 1.73-1. 67 (2H, m), 1.50 (6H, s); MS (ESI) 233 (M + H) +.
References:
WO2005/92858,2005,A2 Location in patent:Page/Page column 142