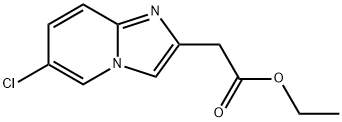
IMidazo[1,2-a]pyridine-2-acetic acid, 6-chloro-, ethyl ester synthesis
- Product Name:IMidazo[1,2-a]pyridine-2-acetic acid, 6-chloro-, ethyl ester
- CAS Number:59128-02-8
- Molecular formula:C11H11ClN2O2
- Molecular Weight:238.67
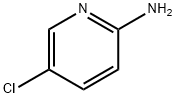
1072-98-6
710 suppliers
$5.00/1g
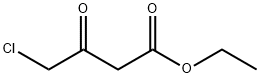
638-07-3
498 suppliers
$5.00/5G
![IMidazo[1,2-a]pyridine-2-acetic acid, 6-chloro-, ethyl ester](/CAS/GIF/59128-02-8.gif)
59128-02-8
39 suppliers
$52.10/250mg
Yield:59128-02-8 86%
Reaction Conditions:
in ethanol at 90; for 15 h;
Steps:
31.A Step A:
Step A: A mixture of 5-chloropyridin-2-amine (2.57 g, 20 mmol) and ethyl 4-chloro- 3-oxobutanoate (3.95 g, 24 mmol) in EtOH (20 mL) was heated at 90 °C for 15 h, then the solvent was removed. The residue was suspended in CH3CN, collected by vacuum filtration, washed with CH3CN and dried to give ethyl 2-(6-chloroimidazo[l,2-a]pyridin-2-yl)acetate (4.14 g, 86%) as a white solid. MS m/z 239.1 [M+H]+.
References:
WO2013/101974,2013,A1 Location in patent:Paragraph 00763; 00764