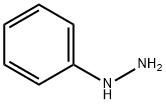
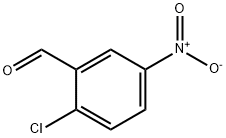
N-(2-CHLORO-5-NITRO-BENZYLIDENE)-N'-PHENYL-HYDRAZINE synthesis
- Product Name:N-(2-CHLORO-5-NITRO-BENZYLIDENE)-N'-PHENYL-HYDRAZINE
- CAS Number:59670-70-1
- Molecular formula:C13H10ClN3O2
- Molecular Weight:275.6904
Yield:59670-70-1 66%
Reaction Conditions:
with anhydrous Sodium acetate;glacial acetic acid in methanol at 20; for 4 h;
Steps:
General experimental procedure for thesynthesis of hydrazones 1a-i
General procedure: In a 100 mL round-bottom flask, the o-chlorinated aromaticaldehyde (5 mmol) and phenylhydrazine (0.540 g, 5 mmol)were dissolved in methanol (25 mL). Glacial acetic acid (0.060 g, 20 mol %) and sodium acetate (0.082 g, 20 mol %)were added, and the solution was stirred for 4 h at room temperature.The resulting mixture was cooled in an ice bath and coldwater (30 mL) was added to give a precipitate. In a Büchnerfunnel, the solid was filtered off and washed with an additionalamount of cold water. After drying the solid in a desiccator, itwas recrystallized from methanol/water 1:1 to give the products1a-i with good purity. The identity of the compounds 1a-iwas confirmed by HRMS analysis (see Supporting InformationFile 1).
References:
Barbosa, Yara Cristina Marchioro;Paveglio, Guilherme Caneppele;de Pereira, Claudio Martin Pereira;Moura, Sidnei;Schwalm, Cristiane Storck;Casagrande, Gleison Antonio;Pizzuti, Lucas [Beilstein Journal of Organic Chemistry,2022,vol. 18,p. 1079 - 1087]