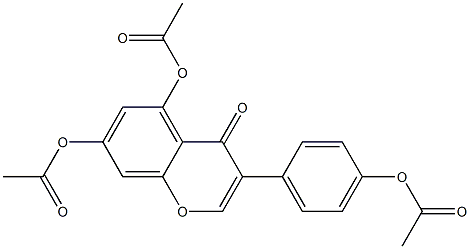
4H-1-Benzopyran-4-one, 5,7-bis(acetyloxy)-3-[4-(acetyloxy)phenyl]- synthesis
- Product Name:4H-1-Benzopyran-4-one, 5,7-bis(acetyloxy)-3-[4-(acetyloxy)phenyl]-
- CAS Number:5995-97-1
- Molecular formula:C21H16O8
- Molecular Weight:396.347
Yield:5995-97-1 98%
Reaction Conditions:
with pyridineReflux;
Steps:
3.4 Acylation of apigenin, luteolin, daidzein and genistein
General procedure: To solution of apigenin (3mmol) in acetic anhydride (6mL) was added pyridine (0.6mL). Then, the mixture was heated to reflux until apigenin consumption and was poured into ice-cold water (70mL) to afford 4 as a white solid. The crude product was recrystallized to get pure compound. Compounds 5, 12 and 13 were synthesized according to the method for 4.
4.1.3.4
4', 5, 7-O-triacetylgenistein (13)
Compound 13 was recrystallized from ethyl acetate.
Yield 98.0%; White solid. 1H NMR (400 MHz, CDCl3) δ 7.89 (s, 1H, H-2), 7.49 (d, 2H, J = 8.0 Hz, H-2', H-6'), 7.25 (d, 1H, J = 2.0 Hz, H-8), 7.15 (d, 2H, J = 8.0 Hz, H-3', H-5'), 6.86 (d, 1H, J = 2.0 Hz, H-6), 2.41 (s, 3H, -OCOCH3), 2.34 (s, 3H, -OCOCH3), 2.31 (s, 3H, -OCOCH3).
References:
Li, Yue-Qing;Yang, Fei;Wang, Liu;Cao, Zhi;Han, Tian-Jiao;Duan, Zhe-Ang;Li, Zhen;Zhao, Wei-Jie [European Journal of Medicinal Chemistry,2016,vol. 112,p. 196 - 208]

152-95-4
228 suppliers
$5.00/50mg
![4H-1-Benzopyran-4-one, 5,7-bis(acetyloxy)-3-[4-(acetyloxy)phenyl]-](/CAS/20180529/GIF/5995-97-1.gif)
5995-97-1
6 suppliers
inquiry

