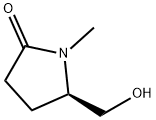
(5R)-5-(hydroxyMethyl)-1-Methyl-2-Pyrrolidinone synthesis
- Product Name:(5R)-5-(hydroxyMethyl)-1-Methyl-2-Pyrrolidinone
- CAS Number:122663-22-3
- Molecular formula:C6H11NO2
- Molecular Weight:129.16

122742-14-7
37 suppliers
$160.00/1g

122663-22-3
25 suppliers
inquiry
Yield:122663-22-3 92.2 g
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran at 0; for 1 h;
Steps:
1.S3 Step three:
Compound IN-2 (132 g, crude product) was added to THF (600 mL), the reaction solution was cooled to 0°C, sodium borohydride (31.8 g, 0.84 mol) was added in batches, and the mixture was stirred at 0°C for 60 minutes.TLC detects the completion of the reaction. At 0°C, 1N HCl (200 mL) was added dropwise to quench the reaction.Water (400 mL) was added to the reaction solution, and extraction was performed twice with dichloromethane (600 mL).The organic phases were combined and washed with saturated brine (600 mL) and water (600 mL) respectively.The organic phase was dried with anhydrous sodium sulfate.After filtration, 142 g of crude product was obtained by distillation under reduced pressure.The crude product was separated by silica gel column (ethyl acetate: petroleum ether = 1:3) to obtain compound IN-3 (92.2 g, 85%).
References:
CN111763206,2020,A Location in patent:Paragraph 0048-0049; 0054-0055

4042-36-8
388 suppliers
$6.00/5g

122663-22-3
25 suppliers
inquiry
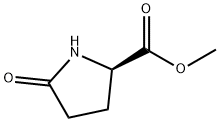
64700-65-8
84 suppliers
$27.50/1g

122663-22-3
25 suppliers
inquiry