
6-(2-hydroxyethoxy)nicotinaldehyde synthesis
- Product Name:6-(2-hydroxyethoxy)nicotinaldehyde
- CAS Number:1011487-86-7
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
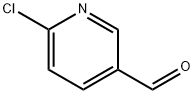
23100-12-1
261 suppliers
$6.00/1g
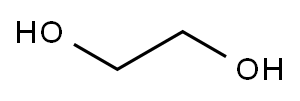
107-21-1
1318 suppliers
$10.00/25g

1011487-86-7
6 suppliers
inquiry
Yield:1011487-86-7 85%
Reaction Conditions:
Stage #1: ethylene glycolwith sodium t-butanolate at 20; for 0.5 h;
Stage #2: 6-chloronicotinylaldehyde at 20 - 80;
Steps:
16.2.1 Step 1:
Synthesis of Compound 6-(2-hydroxyethoxy)nicotinaldehyde
t-BuONa (3.49 g, 36.3 mmol) was added to ethane-1,2-diol (30 mL) at RT.
After stirring for 30 min, 6-chloronicotinaldehyde (4.0 g, 28.3 mmol) was added and the resulting mixture was stirred at RT overnight, then it was heated to 80° C. and stirred for additional 2 hours.
After the reaction was completed, the reaction mixture was cooled to RT, poured into ice-water and extracted with EA.
The organic phase was washed with water, dried and concentrated.
The crude product was separated by column chromatography to give the desired product (4.01 g, yield 85%).
References:
US2020/190066,2020,A1 Location in patent:Paragraph 0300-0301