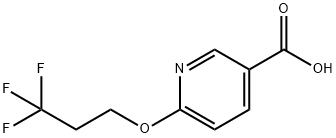
6-(3,3,3-Trifluoropropoxy)nicotinic acid synthesis
- Product Name:6-(3,3,3-Trifluoropropoxy)nicotinic acid
- CAS Number:1072855-39-0
- Molecular formula:C9H8F3NO3
- Molecular Weight:235.16
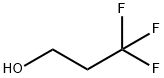
2240-88-2
187 suppliers
$14.00/1g
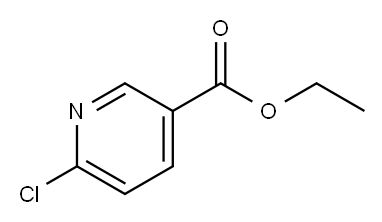
49608-01-7
266 suppliers
$7.00/5g

1072855-39-0
10 suppliers
inquiry
Yield:1072855-39-0 81%
Reaction Conditions:
Stage #1: 3,3,3-Trifluoropropanolwith potassium tert-butylate in tetrahydrofuran at 0; for 0.0833333 h;
Stage #2: 6-chloro-3-pyridinecarboxylic acid ethyl ester in tetrahydrofuran at 20; for 2 h;
Stage #3: with hydrogenchloride;lithium hydroxide;watermore than 3 stages;
Steps:
I-11
Intermediate Example, 1-11Acid 30: 6-(3,3,3-Trifluoropropoxy)nicotinic acidTo a solution of potassium tert-butoxide (0.864 g, 7.7 mmol) in tetrahydrofuran (10 mL) 3,3,3-trifluoropropan-l-ol (0.878 g, 7.7 mmol) was added at 0 0C. After 5 min ethyl 6- chloronicotinate (1.3 g, 7.0 mmol) was added to the stirred solution. The mixture was allowed to reach ambient temperature and stirred for an additional 2 h. Brine was added and the mixture was extracted with ethyl acetate. The organic phase was dried over sodium sulfate, filtered and concentrated in vacuo. The residue (1.26 g, 4.8 mmol) was dissolved in a mixture of tetrahydrofuran (4 mL) and water (1 mL) and treated with lithium hydroxide (0.126 g, 3.0 mmol). The mixture was stirred at ambient temperature for 16 h and the tetrahydrofuran was removed in vacuo. Water (5 mL) was added and the pH adjusted to 2 with hydrochloric acid (4 M). The resulting precipitate was collected by filtration, washed with water and dried in vacuo to give 0.913 g (81% yield) of the title compound: 1H NMR (DMSO-J6) δ 8.72 (d, 1 H), 8.16 (dd, 1 H), 6.91 (d, 1 H), 4.56 (t, 2 H), 2.81 (dd, 2 H); MS (ESI) m/z 236 [M+H]+.
References:
WO2008/130321,2008,A2 Location in patent:Page/Page column 47

73781-91-6
276 suppliers
$5.00/1g

1072855-39-0
10 suppliers
inquiry