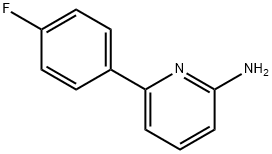
6-(4-FLUOROPHENYL)PYRIDIN-2-AMINE synthesis
- Product Name:6-(4-FLUOROPHENYL)PYRIDIN-2-AMINE
- CAS Number:1159819-35-8
- Molecular formula:C11H9FN2
- Molecular Weight:188.2
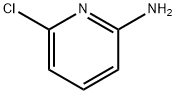
45644-21-1
357 suppliers
$10.00/5g
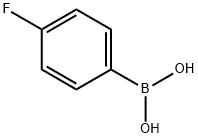
1765-93-1
551 suppliers
$9.00/1g

1159819-35-8
7 suppliers
inquiry
Yield:1159819-35-8 68%
Reaction Conditions:
with potassium phosphate;1,1'-bis(di-tertbutylphosphino)ferrocene;palladium diacetate in 1,4-dioxane; for 4 h;Inert atmosphere;Reflux;
Steps:
8.a
a) 6-(4-Fluoro-phenyl)-pyridin-2-ylamine; 2-Amino-6-chloropyridine (128.6 mg, 1 mmol), 4-fluorobenzeneboronic acid (216.4 mg, 1.5 mmol) and potassium phosphate tribasic (433.2 mg, 2 mmol) were suspended in dioxane (3.9 mL) The suspension was evacuated and flushed with nitrogene for 3 times. Palladium (II) acetat (11.2 mg, 0.05 mmol) and (D-t-BPF) (1,1'-bis(di-tert.-butylphosphino) ferrocene, (24.2 mg, 0.05 mmol) were added and again the suspension was evacuated and flushed with nitrogen twice. The reaction was heated to reflux for 4 hours. After cooling to room temperature the reaction was partitionated between water and EtOAc and extracted once with EtOAc. The combined organic layers were washed with 1N aqueous NaOH solution and once with saturated aqueous NaCl solution, dried over sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel chromatography using CH2Cl2/MeOH (v/v 19:1) as eluent to yield the title compound as a light brown oil (142 mg, 68%).MS ISP (m/e): 189.4 (59) [(M+H)+], 173.2 (100).1H NMR (DMSO-D6, 300 MHz): δ (ppm)=8.01 (m, 2H), 7.45 (t, 1H), 7.24 (t, 2H), 7.03 (d, 1H), 6.41 (d, 1H), 5.98 (br s, 2H).
References:
US2011/190269,2011,A1 Location in patent:Page/Page column 20