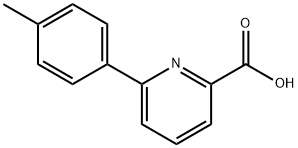
6-(4-Methylphenyl)-picolinic acid synthesis
- Product Name:6-(4-Methylphenyl)-picolinic acid
- CAS Number:86696-72-2
- Molecular formula:C13H11NO2
- Molecular Weight:213.23
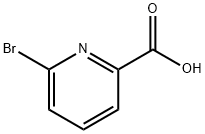
21190-87-4
385 suppliers
$10.00/5g
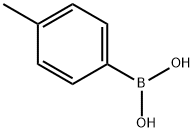
5720-05-8
429 suppliers
$5.00/1g

86696-72-2
19 suppliers
$74.00/100mg
Yield:86696-72-2 90%
Reaction Conditions:
Stage #1: 6-bromo-pyridine-2-carboxylic acid;4-methylphenylboronic acidwith sodium hydrogencarbonate;tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;water at 100; for 2 h;Microwave;
Stage #2: with hydrogenchloride in water; pH=1;
Steps:
A1.2.A
Step A. 6-p-Tolyl-pyridine-2-carboxylic acid. A mixture of 6-bromo-pyridine-2-carboxylic acid (202 mg, 1 mmol), 4-methylphenylboronic acid (163 mg, 1.2 mmol), and Pd(PPh3)4 (25 mg) in saturated aq. NaHCO3 solution (3 mL) and DME (3 mL) was irradiated in a microwave on a Biotage Smith Synthesizer at 100° C. for 2 hrs. TLC showed the reaction was completed. The mixture was filtered and washed with water (2*50 mL) and ether (2*50 mL). The organic and aqueous layers were separated. The pH of the aqueous portion was adjusted to about 1 with 1 N aq. HCl solution. The aqueous layer was then extracted with ethyl acetate (3*60 mL). The combined organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was evaporated to give the title compound (191 mg, 90% yield).
References:
US2012/4198,2012,A1 Location in patent:Page/Page column 33-34
76781-76-5
0 suppliers
inquiry

86696-72-2
19 suppliers
$74.00/100mg
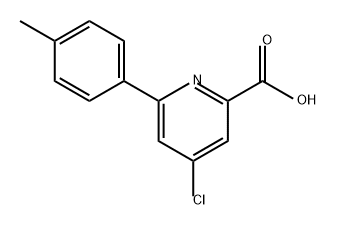
86696-47-1
0 suppliers
inquiry

86696-72-2
19 suppliers
$74.00/100mg