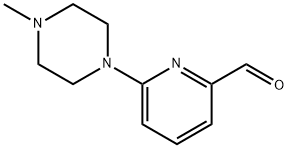
6-(4-Methylpiperazin-1-yl)picolinaldehyde synthesis
- Product Name:6-(4-Methylpiperazin-1-yl)picolinaldehyde
- CAS Number:1216691-10-9
- Molecular formula:C11H15N3O
- Molecular Weight:205.26

109-01-3
652 suppliers
$5.00/5G
34160-40-2
341 suppliers
$6.00/1g

1216691-10-9
8 suppliers
$360.00/1g
Yield:1216691-10-9 32%
Reaction Conditions:
with potassium carbonate in acetonitrile at 110; for 17 h;Sealed tube;
Steps:
15
Example 15
5,7-Dimethoxy-2-(6-(4-methylpiperazin-1-yl)pyridin-2-yl)quinazolin-4(3M-one Hydrochloride
Preparation of 6-(4-Methylpiperazin-1-yl)picolinaldehyde (3).
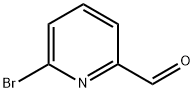
A suspension of 6-bromopicolinaldehyde (1.50 g, 8.10 mmol), 1-methylpiperazine (2, 4.04 g, 40.0 mmol), potassium carbonate (4.46 g, 32.0 mmol) and anhydrous CH3CN (10 mL) was placed in a sealed vessel and the mixture was heated at 110° C. for 17 h.
The mixture was diluted with water (50 mL) and brought to pH 7 using 2 N aq. HCl (15 mL).
The resulting solution was extracted with CH2Cl2 (3*50 mL) and the combined extracts were washed with brine (200 mL).
The solution was dried (Na2SO4), filtered, concentrated and purified by silica gel chromatography eluting with 0-5% CH3OH in CH2Cl2 to afford the title compound (0.528 g, 32%) as an orange oil: 1H NMR (500 MHz, CDCl3) δ 9.84 (s, 1H), 7.62 (t, J=8.0 Hz, 1H), 7.27 (d, J=7.0 Hz, 1H), 6.85 (d, J=7.0 Hz, 1H), 3.67-3.65 (m, 4H), 2.55-2.52 (m, 4H), 2.36 (s, 3H).
References:
US2014/142102,2014,A1 Location in patent:Paragraph 0549; 0550