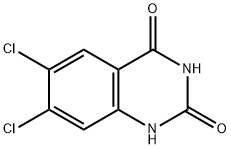
6,7-DICHLOROQUINAZOLINE-2,4(1H,3H)-DIONE synthesis
- Product Name:6,7-DICHLOROQUINAZOLINE-2,4(1H,3H)-DIONE
- CAS Number:864293-02-7
- Molecular formula:C8H4Cl2N2O2
- Molecular Weight:231.04
Yield:864293-02-7 94%
Reaction Conditions:
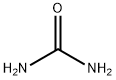
Stage #1: 2-amino-4,5-dichloro-benzoic acid;urea at 160; for 6 h;
Stage #2: with sodium hydroxide in water; for 0.0833333 h;Heating / reflux;
Steps:
C
General method C: Synthesis of quinazoline-diones from their corresponding anthranilic acid precursors. The following procedure described for 6,7-dichloroquinazolin-2,4(1H,3H-dione is representative for the synthesis of intermediate quinazolinediones.; [Show Image] 6,7-dichloroquinazolin-2,4(1H,3H)-dione; 2-Amino-4,5-dichloro benzoic acid (920 mg, 4.58 mmol) and urea (2.75 g, 45.8 mmol) were stirred at 160°C. After 6 hours the mixture was cooled to 100°C and an equivalent volume of water was added while stirring was continued for 5 minutes. The formed precipitate was filtered off and washed with water to yield a solid cake that was suspended in a solution of 0.5 N NaOH in water. The suspension was heated to boil for 5 minutes and then cooled to r.t. The pH was adjusted to about 2 with HCI and the quinazoline-dione was filtered off. After washing with a mixture of water and methanol the product was dried in vacuo to yield 994 mg (94%) of a solid. 1H-NMR (DMSO-δ6) δ (ppm) 7.89 (s,1H), 7.33 (s,1H).
References:
EP2077263,2009,A1 Location in patent:Page/Page column 17