
6,8-dichloro-2,7-naphthyridin-1-ol synthesis
- Product Name:6,8-dichloro-2,7-naphthyridin-1-ol
- CAS Number:950746-21-1
- Molecular formula:C8H4Cl2N2O
- Molecular Weight:215.04
![3-Pyridinecarbonitrile, 2,6-dichloro-4-[(1E)-2-(dimethylamino)ethenyl]-](/CAS/20200611/GIF/1175559-53-1.gif)
1175559-53-1

950746-21-1
Step 4: To a sealed reaction tube was added 2,6-dichloro-4-[(1E)-2-(dimethylamino)vinyl]pyridine-3-carbonitrile (4.0 g, 16.6 mmol) and concentrated hydrochloric acid (20 mL). The reaction mixture was stirred at 45 °C for 18 hours. After completion of the reaction, it was cooled to room temperature and ice water was added to the reaction solution to form a yellow slurry. The precipitate was collected by filtration, washed sequentially with cold water, ether and ethyl acetate, and then dried under vacuum to afford 6,8-dichloro-2,7-naphthyridin-1(2H)-one as a pale yellow solid (80% yield). Mass spectrum (MS) m/z: 215.0 (M + 1). 1H NMR (300 MHz, DMSO-d6): δ 11.75 (s, 1H), 7.76 (s, 1H), 7.50 (t, J = 6.6 Hz, 1H), 6.52 (d, J = 6.6 Hz, 1H).
![3-Pyridinecarbonitrile, 2,6-dichloro-4-[(1E)-2-(dimethylamino)ethenyl]-](/CAS/20200611/GIF/1175559-53-1.gif)
1175559-53-1
3 suppliers
inquiry

950746-21-1
41 suppliers
$217.00/100mg
Yield:950746-21-1 80%
Reaction Conditions:
with hydrogenchloride in water at 45; for 18 h;Sealed tube;
Steps:
1.4
Step 4: 2,6-dichloro-4-((E)-2-(dimethylamino)vinyl)pyridine-3-carbonitrile (4.0g, 16.6mmol) was added with 20mL concentrated HCl in a sealed tube. The reaction is stirred at 45°Cfor 18h. After cooling down the reaction to RT, ice water was added to the solution resulting heavy yellow slurry. The precipitate was collected by filtration, washed with cold water, ether and ethyl acetate, and dried under vacuum to get light yellow solid 6,8-dichloro-2,7-naphthyridin-l(2H)-one (yield -80%). MS m/z 215.0 (M + 1). 'HNMR (300 MHz, DMSO-i 6): 51 1.75 (s, 1H), 7.76 (s, 1H), 7.50 (t, J=6.6Hz, 1H), 6.52 (d, J=6.6Hz, 1H).
References:
WO2013/185353,2013,A1 Location in patent:Page/Page column 21
![3-Pyridinecarbonitrile, 2,6-dichloro-4-[2-(1-pyrrolidinyl)ethenyl]-](/CAS/20210305/GIF/950746-20-0.gif)
950746-20-0
0 suppliers
inquiry

950746-21-1
41 suppliers
$217.00/100mg
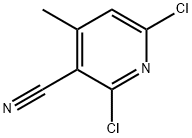
875-35-4
269 suppliers
$13.00/25g

950746-21-1
41 suppliers
$217.00/100mg
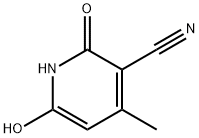
5444-02-0
203 suppliers
$10.00/5g

950746-21-1
41 suppliers
$217.00/100mg