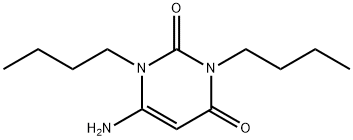
6-AMino-1,3-dibutyluracil synthesis
- Product Name:6-AMino-1,3-dibutyluracil
- CAS Number:41862-16-2
- Molecular formula:C12H21N3O2
- Molecular Weight:239.31

619-37-4
6 suppliers
inquiry

372-09-8
356 suppliers
$20.00/25g
![Acetamide, N-butyl-N-[(butylamino)carbonyl]-2-cyano-](/CAS/20210305/GIF/161419-76-7.gif)
161419-76-7
0 suppliers
inquiry

41862-16-2
16 suppliers
$125.00/250mg
Yield:41862-16-2 99.5%
Reaction Conditions:
with sodium hydroxide;acetic anhydride in methanol;
Steps:
19 Example 19
Example 19 This example describes the synthesis of 1,3-di-n-butyl-6-aminouracil. N,N-Di-n-butylurea (34.45 g, 0.2 mmol), cyanoacetic acid (19.56 g, 0.23 mol), and acetic anhydride (81.66 ml, 0.8 mol) were heated at 80° C. for 2 h under nitrogen and solvent was removed by rotary evaporation to yield N-(2-cyanoacetyl)-N,N'-dibutylurea. The N-(2-cyanoacetyl)-N,N'-dibutylurea was dissolved in methanol (100 ml) and 4N NaOH (100 ml) was added. The reaction mixture was cooled for 30 min with stirring and the solid was filtered and dried to yield 1,3-di-n-butyl-6-aminouracil (47.04 g, 99.5%) as a colorless solid, m.p. 90.1°-92.4° C.; 1 H NMR (DMSO-d6) δ1.72-1.93 (m, 6H, 2*CH3, 1.18-1.33 (m, 4H, 2*CH2), 1.39-1.52 (M, 4H, 2*CH2), 3.70 (t, 2H, N--CH2), 3.76 (t, 2H, N--CH2), 4.64 (s, 1H, H-4), 6.77 (br s, exchangeable with D2 O, 2H, NH2).
References:
US5773423,1998,A
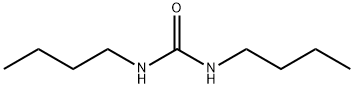
1792-17-2
99 suppliers
$22.00/5g

372-09-8
356 suppliers
$20.00/25g

41862-16-2
16 suppliers
$125.00/250mg
![Acetamide, N-butyl-N-[(butylamino)carbonyl]-2-cyano-](/CAS/20210305/GIF/161419-76-7.gif)
161419-76-7
0 suppliers
inquiry

41862-16-2
16 suppliers
$125.00/250mg

1792-17-2
99 suppliers
$22.00/5g

41862-16-2
16 suppliers
$125.00/250mg
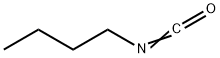
111-36-4
200 suppliers
$41.00/5ml

41862-16-2
16 suppliers
$125.00/250mg