
6-Amino-5-nitro-pyridine-2-carbonitrile synthesis
- Product Name:6-Amino-5-nitro-pyridine-2-carbonitrile
- CAS Number:516481-67-7
- Molecular formula:C6H4N4O2
- Molecular Weight:164.12

84487-04-7
125 suppliers
$31.00/200μmol

516481-67-7
8 suppliers
inquiry
Yield:516481-67-7 50 mg (67%)
Reaction Conditions:
in dichloromethane;N,N-dimethyl acetamide;
Steps:
75.C 2-[4-(3,4-Dichloro-phenoxy)-phenyl]-1H-imidazo[4,5-b]pyridine-5-carboxylic Acid Amide.
C. 6-Amino-5-nitro-pyridine-2-carbonitrile. To a glass pressure vessel was added 6-bromo-3-nitro-pyridin-2-ylamine (100 mg, 0.46 mmol), copper cyanide (123 mg, 1.38 mmol) and N,N-dimethylacetamide (3.0 mL). The vessel was sealed, and the contents were heated to 120° C. for 24 h. The reaction mixture was cooled to room temperature and diluted with water (10 mL). The resulting precipitate was collected by filtration and was then stirred with CH2Cl2 (10 mL) for 24 h. The mixture was filtered, and the filtrate was concentrated to afford 50 mg (67%) of 6-amino-5-nitro-pyridine-2-carbonitrile. HPLC: Rt=7.63. 1H NMR (400 MHz, DMSO-d6): 8.68 (d, J=8.4 Hz, 1H), 8.27 (br s, 2H), 7.34 (d, J=8.4 Hz, 1H).
References:
US2003/176438,2003,A1
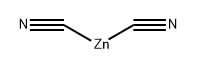
557-21-1
101 suppliers
$12.00/5g

27048-04-0
340 suppliers
$5.00/250mg

516481-67-7
8 suppliers
inquiry
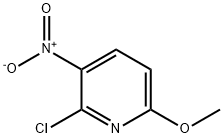
38533-61-8
300 suppliers
$8.00/5g

516481-67-7
8 suppliers
inquiry
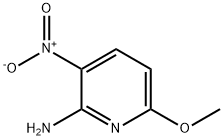
73896-36-3
232 suppliers
$9.00/10g

516481-67-7
8 suppliers
inquiry
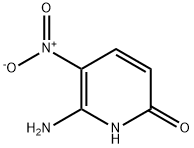
211555-30-5
46 suppliers
$58.00/100mg

516481-67-7
8 suppliers
inquiry