6-aminopyrimidine-4-carbonitrile synthesis
- Product Name:6-aminopyrimidine-4-carbonitrile
- CAS Number:1353100-84-1
- Molecular formula:C5H4N4
- Molecular Weight:120.11
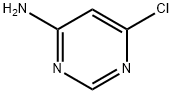
557-21-1
86 suppliers
$12.00/5g
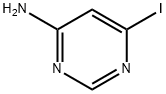
53557-69-0
66 suppliers
$60.00/25mg

1353100-84-1
10 suppliers
inquiry
Yield:1353100-84-1 88%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in 1-methyl-pyrrolidin-2-one at 80; for 2 h;Inert atmosphere;
Steps:
6.1.2 2-Aminopyrimidine-4-carbonitrile (57)
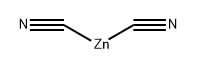
General procedure: A flask containing a solution of 55-iodide (4.09g, 18.5mmol) in NMP (75ml) was purged with argon for 5min. Zinc cyanide (2.28g, 19.4mmol) and tetrakis(triphenylphosphine)palladium (0) (1.71g, 1.48mmol) were added and the mixture was stirred at 80°C for 2h. The mixture was cooled to room temperature, EtOAc (200ml) and 30% aqueous NH4OH (200ml) was added, and stirring was continued for 1h. The layers were separated, the aqueous layer was extracted with EtOAc (4×200ml), and the combined organic layers were concentrated under reduced pressure. Et2O (30ml) was added and the precipitate was collected by filtration and washed with Et2O to deliver 2-aminopyrimidine-4-carbonitrile (1.66g, 13.8mmol, 75% yield) as a white solid.
References:
Reichelt, Andreas;Bailis, Julie M.;Bartberger, Michael D.;Yao, Guomin;Shu, Hong;Kaller, Matthew R.;Allen, John G.;Weidner, Margaret F.;Keegan, Kathleen S.;Dao, Jennifer H. [European Journal of Medicinal Chemistry,2014,vol. 80,p. 364 - 382]

557-21-1
86 suppliers
$12.00/5g

1159818-57-1
78 suppliers
$13.00/100mg

1353100-84-1
10 suppliers
inquiry