
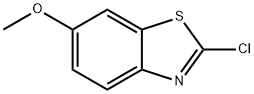
6-Benzothiazolol,2-chloro-(7CI,9CI) synthesis
- Product Name:6-Benzothiazolol,2-chloro-(7CI,9CI)
- CAS Number:2591-16-4
- Molecular formula:C7H4ClNOS
- Molecular Weight:185.63
2605-14-3
161 suppliers
$39.00/1g

2591-16-4
38 suppliers
$45.00/10mg
Yield:2591-16-4 95%
Reaction Conditions:
with boron trichloride;tetra-(n-butyl)ammonium iodide in n-heptane;dichloromethane at -78 - 20;
Steps:
144 Intermediate 1 44A: 2-chlorobenzo[djthiazol-6-ol
To 2-chloro-6-methoxybenzo[djthiazole (8.4 g, 42.1 mmol) andtetrabutylammonium iodide (16.32 g, 44.2 mmol) in dichloromethane (150 ml) at -78 °Cwas added 1.0 M boron trichloride in heptane (99 ml, 99 mmol) dropwise. The mixturewas slowly warmed up by removing the cooling bath and stirred at room temperature overnight. HPLC and LCMS indicated a clean reaction. The mixture was poured into 1.5 M potassium phosphate and ice, stirred for 20 mm, extracted with EtOAc. The organic layers were collected, washed with 10% Na25203, water, brine and dried over sodiumsulfate. The crude product was purified by flash chromatography (loading in chloroform/THF, 5% to 60% EtOAc in hexane over 15 mm using a 220 g silica gel cartridge). The desired fractions were combined and concentrated to yield Intermediate 144A (7.4 g, 39.9 mmol, 95 % yield) as a white sold. ‘H NMR (400MHz, METHANOL-d4) 7.70 (d, J=8.8 Hz, 1H), 7.25 (d, J=2.4 Hz, 1H), 7.00 (dd, J8.9, 2.5Hz, 1H).
References:
WO2018/13776,2018,A1 Location in patent:Page/Page column 331