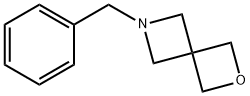
6-benzyl-2-oxa-6-azaspiro[3.3]heptane synthesis
- Product Name:6-benzyl-2-oxa-6-azaspiro[3.3]heptane
- CAS Number:46246-91-7
- Molecular formula:C12H15NO
- Molecular Weight:189.25
Yield: 70%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethyl-formamide at 60; for 18 h;Temperature;Reagent/catalyst;Solvent;
Steps:
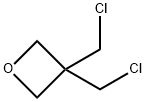
6-Benzyl-2-oxa-6-azaspiro[3.3]heptane (6)
In a 2 L reaction flask, bis(bromomethyl)oxetane (5b; 129 g, 518mmol) was dissolved in anhyd DMF (1300 mL). At r.t., benzylamine(61.1 g, 570 mmol) and DBU (166 g, 1088 mmol) were added. The reaction mixture was stirred for 18 h at 60 °C at which point, GC analysisshowed clean conversion into 6. The reaction mixture was poured into a stirring mixture of MTBE/EtOAc (1 L:1 L) and brine/H2O (1 L:0.5L). The aqueous phase was extracted twice with MTBE/EtOAc (1:1,800 mL and 400 mL) and with EtOAc (2 × 250 mL). The combined organic phases were washed with brine, dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by a short-path distillation (96 °C/0.02 mbar) providing 69 g (70%) of 6 as a colorless oil. 1H NMR (CDCl3, 400 MHz): δ = 7.33-7.22 (m, 5 H), 4.74 (s, 4 H), 3.54(s, 2 H), 3.36 (s, 4 H).13C NMR (DMSO-d6, 100 MHz): δ = 138.3 (C), 128.2 (CH), 128.1 (CH),126.8 (CH), 80.0 (CH2), 62.9 (CH2), 62.5 (CH2), 38.5 (C).MS (EI, 70 eV): m/z (%) = 91.1 (100, [Bn]+·), 188 (10, [M - 1]+·), 189 (7,[M]+·).
References:
Van Der Haas, Richard N. S.;Dekker, Jeroen A.;Hassfeld, Jorma;Hager, Anastasia;Fey, Peter;Rubenbauer, Philipp;Damen, Eric [Synthesis,2017,vol. 49,# 11,art. no. SS-2016-T0855-PSP,p. 2394 - 2401]
1522-92-5
238 suppliers
$6.00/25g
![6-benzyl-2-oxa-6-azaspiro[3.3]heptane](/CAS/20150408/GIF/46246-91-7.gif)
46246-91-7
45 suppliers
inquiry