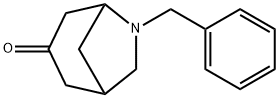
6-benzyl-6-azabicyclo[3.2.1]octan-3-one synthesis
- Product Name:6-benzyl-6-azabicyclo[3.2.1]octan-3-one
- CAS Number:16607-47-9
- Molecular formula:C14H17NO
- Molecular Weight:215.29
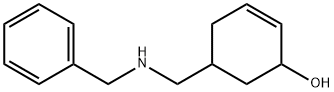
61088-57-1
3 suppliers
inquiry
![6-benzyl-6-azabicyclo[3.2.1]octan-3-one](/CAS/20180703/GIF/16607-47-9.gif)
16607-47-9
15 suppliers
inquiry
Yield:16607-47-9 68%
Reaction Conditions:
with manganese(IV) oxide in dichloromethane; for 2 h;
Steps:
1 6-Benzyl-6-azabicyclo [3.2. 1] octan-3-one
To a solution of 5- (benzylaminomethyl) cyclohex-2-enol (3.80 g, 17.5 mmol) in dry dichloromethane (150 ml) was added activated manganese dioxide (18.0 g, 210 mmol) in one portion. The mixture was stirred vigorously under nitrogen for 2 h. The yellow solution was filtered through Celite and the solids were washed with dichloromethane (2 x 50 ml). The combined filtrates were concentrated by rotary evaporation to give an orange oil, which solidified on brief standing. The solid was recrystallized from hot hexane/ether to afford the ketone as an off-white solid (2.6 g, 68%)
References:
WO2005/37832,2005,A2 Location in patent:Page/Page column 68