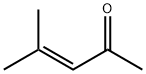
6-bromo-1,2-dihydro-2,2,4-trimethylquinoline synthesis
- Product Name:6-bromo-1,2-dihydro-2,2,4-trimethylquinoline
- CAS Number:91720-32-0
- Molecular formula:C12H14BrN
- Molecular Weight:252.15
Yield:91720-32-0 62%
Reaction Conditions:
with bismuth(lll) trifluoromethanesulfonate for 72 h;Reflux;Inert atmosphere;Skraup Quinoline Synthesis;
Steps:
4.4.1 6-Bromo-2,2,4-trimethyl-1,2-dihydroquinoline (13)
Bismuth(III) trifluoromethanesulfonate (19.0g, 30.0mmol) was added to a solution of 4-bromoaniline (25.0g, 145mmol) in acetone (500mL), and the mixture was stirred at reflux for 3days. The solvent was removed under vacuum, and the residue was partitioned between ether (300mL) and water (200mL). The layers were separated, and the aqueous phase was extracted with ether (2×100mL). The combined organic extracts were washed with saturated NaCl and evaporated under vacuum. The crude product was purified by column chromatography (ether in hexanes gradient) to afford 13 as a brown solid (23g, 89.9mmol, 62%), mp 83-85°C. IR: 3382, 1486, 1257, 806cm-1; 1H NMR (400MHz, CDCl3): δ 7.13 (d, J=2.2Hz, 1H, ArH), 7.05 (dd, J=8.4, 2.2Hz, 1H, ArH), 6.31 (d, J=8.4Hz, 1H, ArH), 5.33 (s, 1H, =CH), 3.71 (br s, 1H, NH), 1.95 (s, 3H, =CCH3), 1.26 (s, 6H, C(CH3)2); 13C NMR (101MHz, CDCl3): δ 142.2, 130.7, 129.4, 127.6, 126.2, 123.4, 114.3, 108.6, 51.9, 30.9, 18.4.
References:
Gnanasekaran, Krishna Kumar;Pouland, Tim;Bunce, Richard A.;Darrell Berlin;Abuskhuna, Suaad;Bhandari, Dipendra;Mashayekhi, Maryam;Zhou, Donghua H.;Benbrook, Doris M. [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 1,art. no. 115244]

106-40-1
458 suppliers
$12.00/5g

91720-32-0
25 suppliers
inquiry
76-09-5
376 suppliers
$10.00/1g
586-78-7
9 suppliers
$14.00/5g

91720-32-0
25 suppliers
inquiry

106-40-1
458 suppliers
$12.00/5g