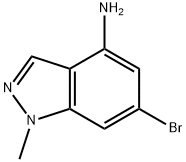
6-Bromo-1-methyl-1H-indazol-4-amine synthesis
- Product Name:6-Bromo-1-methyl-1H-indazol-4-amine
- CAS Number:1198438-39-9
- Molecular formula:C8H8BrN3
- Molecular Weight:226.07

885518-50-3
103 suppliers
$45.00/250mg

74-88-4
344 suppliers
$15.00/10g

1198438-39-9
5 suppliers
inquiry
Yield:1198438-39-9 48 mg
Reaction Conditions:
Stage #1: 6-bromo-1H-indazole-4-aminewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.25 h;
Stage #2: methyl iodide in tetrahydrofuran;mineral oil at 0; for 3 h;
Steps:
6-Bromo-1-methyl-1H-indazol-4-amine
6-Bromo-1-methyl-1H-indazol-4-amine
6-Bromo-1H-indazol-4-amine (available from Sinova, 300 mg, 1.42 mmol) was dissolved in THF (7.5 ml) and the mixture cooled to 0° C. Sodium hydride (60% in mineral oil) (62 mg) was then slowly added.
The mixture was stirred for 15 min, then methyl iodide (221 mg) was added and stirring continued at 0° C. for 3 h.
The reaction mixture was quenched by careful addition of methanol (2 ml), then water (10 ml), then extracted into ethyl acetate and the organic layer was concentrated in vacuo.
The residue was purified by column chromatography on silica eluting with a gradient of 0-50% ethyl acetate in cyclohexane.
Fractions containing desired product were combined and concentrated in vacuo to afford the title compound, 48 mg. LCMS (Method E): R=0.91 mi MH=227.
References:
US9326987,2016,B2 Location in patent:Page/Page column 155; 156

885518-46-7
133 suppliers
$39.00/500mg

1198438-39-9
5 suppliers
inquiry
95192-64-6
103 suppliers
$19.00/250mg

1198438-39-9
5 suppliers
inquiry

606-20-2
5 suppliers
$13.00/5g

1198438-39-9
5 suppliers
inquiry