
6-Bromo-1H-indazol-3-ol synthesis
- Product Name:6-Bromo-1H-indazol-3-ol
- CAS Number:885521-92-6
- Molecular formula:C7H5BrN2O
- Molecular Weight:213.03
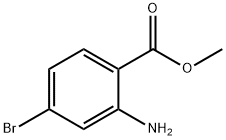
135484-83-2
177 suppliers
$6.00/1g

885521-92-6
79 suppliers
$55.00/250mg
Yield:885521-92-6 50%
Reaction Conditions:
Stage #1: 2-amino-4-bromo-benzoic acid methyl esterwith hydrogenchloride;sodium nitrite in water at 20; for 1 h;
Stage #2: with sodium sulfite in water at 20; for 2 h;
Stage #3: with hydrogenchloride in water at 80; for 18 h;
Steps:
5.1.21. Ethyl 6-bromo-3-oxo-2,3-dihydro-1H-indazole-1-carboxylate (15)
Concentrated hydrochloric acid (1.4 mL, 17 mmol) was added to asuspension of 14 (1.0 g, 4.3 mmol) in H2O (6.7 mL) while cooling onice. Sodium nitrite (0.30 g, 4.4 mmol) in H2O (1.1 mL) was subsequentlyadded to the mixture at the same temperature. After stirring atroom temperature for 1 h, disodium sulfite (1.5 g, 12 mmol) in H2O(6.7 mL) was added and the mixture was stirred at room temperaturefor 2 h. Concentrated hydrochloric acid (2.2 mL, 27 mmol) was addedand the mixture was stirred overnight at room temperature and then foran additional 18 h at 80 °C. After cooling to room temperature, themixture was neutralized with aqueous NaOH solution and the resultingprecipitate was filtered and washed with CHCl3 to give 6-bromo-1,2-dihydro-3H-indazol-3-one as a brown solid (0.47 g, 50% yield). Ethylcarbonochloridate (0.46 g, 4.2 mmol) was added to a solution of thiscompound (0.45 g, 2.1 mmol) in pyridine (1.8 mL) while cooling on ice,and the mixture was stirred at 110 °C for 2 h. The reaction mixture wasconcentrated under reduced pressure, water was added and the resultingprecipitate was filtered and washed with water to give theproduct as a solid (0.50 g, 83% yield). 1H NMR (DMSO-d6) δ ppm 1.36(3H, t, J = 7.1 Hz), 4.42 (2H, q, J = 7.2 Hz), 7.51 (1H, dd, J = 8.5,1.7 Hz), 7.68-7.72 (1H, m), 8.18-8.21 (1H, m), 12.29 (1H, br s); MS(ESI) m/z [M + H]+ 287.
References:
Akashiba, Hiroki;Honda, Shugo;Mitani, Yasuyuki;Sekioka, Ryuichi;Yamasaki, Shingo;Yarimizu, Junko [Bioorganic and medicinal chemistry,2020]