
6-Bromo-2,3-dihydro-1H-inden-5-amine synthesis
- Product Name:6-Bromo-2,3-dihydro-1H-inden-5-amine
- CAS Number:53474-09-2
- Molecular formula:C9H10BrN
- Molecular Weight:212.09
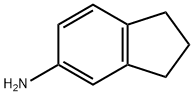
24425-40-9
201 suppliers
$55.00/10g

53474-09-2
9 suppliers
inquiry
Yield:53474-09-2 68%
Reaction Conditions:
with N-Bromosuccinimide in N-methyl-acetamide;
Steps:
1.A A.
A. Formula 2 where X is Bromo 5-Aminoindan (3.0 g, 22.5 mmol) was dissolved in 10 ml of anhydrous dimethylformamide. A solution of N-bromosuccinimide (4.0 g, 22.5 mmol) in 10 ml anhydrous dimethylformamide was added, and the reaction mixture was stirred at room temperature for 1.5 hours. The reaction mixture was partitioned between ethyl acetate and water, and the separated organic layer washed twice with H2 O, then with brine. Drying over Na2 SO4 followed by filtration and evaporation of solvent gave a dark brown oil as the crude product. Purification by flash chromatography (50% EtOAc/hexane) provided 5-amino-6-bromoindan (3.26 g, 68% yield), a compound of Formula 2 where X is bromo, as a brown oil which crystallized into a brown solid. Analytical data: mp 42°-44° C.; 1 H NMR (300 MHz, CDCl3) δ 7.25 (s, 1H), 6.67 (s, 1H), 3.92 (bs, 2H), 2.82-2.75 (m, 4H), 2.08-2.00 (m, 2H); 13 C NMR (300 MHz, CDCl3) δ 144.92; 142.04, 135.77, 127.88, 111.86, 107.11, 32.70, 31.94, 25.86; MS (CI--NH3) m/z 211; Elem. analysis calcd. for C9 H10 BrN: C, 50.97; H, 4.75; N, 6.60; Found: C, 51.10; H, 4.70; N, 6.61.
References:
US5475003,1995,A
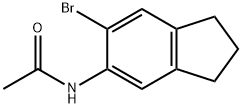
157701-33-2
33 suppliers
$198.00/100mg

53474-09-2
9 suppliers
inquiry
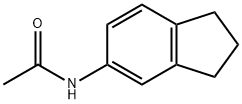
59856-06-3
44 suppliers
$60.00/1g

53474-09-2
9 suppliers
inquiry