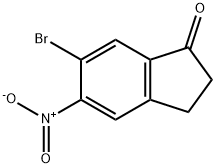
6-bromo-2,3-dihydro-5-nitro-1H-Inden-1-one synthesis
- Product Name:6-bromo-2,3-dihydro-5-nitro-1H-Inden-1-one
- CAS Number:158205-20-0
- Molecular formula:C9H6BrNO3
- Molecular Weight:256.05
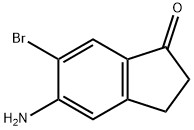
158205-19-7
35 suppliers
$47.00/100mg

158205-20-0
5 suppliers
inquiry
Yield:158205-20-0 47%
Reaction Conditions:
Stage #1: 5-Amino-6-bromo-1-indanonewith tetrafluoroboric acid;sodium nitrite in water at 0; for 0.916667 h;
Stage #2: with copper in water at 26; for 0.5 h;
Steps:
499.E Step E: 6-Bromo-5-nitro-indan-1-one
To a suspension of 5-amino-6-bromo-indan-1-one (4.0 g, 17.7 mmol) in 20% aqueous HBF4 (16 ml) at 0 oC was added 4 M aqueous sodium nitrite (1.9 g, 27.3 mmol) drop wise over a period of 5 min. The mixture was stirred for 50 min after the addition was completed. The resulting foamy suspension was added portion wise to a vigorously stirred mixture of copper (5.3 g, 83.2 mmol) and sodium nitrite (16.3 g, 236.7 mmol) in water (32 ml) at 26oC over a period of 30 min. During the addition, excessive foaming was broken up by the addition of small amounts of diethyl ether. After the mixture was stirred for a further 50 min, it was filtered through Celite pad and washed with ethyl acetate (300 ml). The organic phase was separated, washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by flash column chromatography (10-20% ethyl acetate in petroleum ether) to afford 6-bromo-5-nitro-indan-1-one (2.1 g, 47 %) as a yellow solid.1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.85 (s, 1H), 3.21 (t, J = 6.0 Hz, 2H), 2.83 (t, J = 6.0 Hz, 2H).
References:
WO2017/108723,2017,A2 Location in patent:Page/Page column 749; 750
24425-40-9
206 suppliers
$55.00/10g

158205-20-0
5 suppliers
inquiry
157701-33-2
32 suppliers
$198.00/100mg

158205-20-0
5 suppliers
inquiry
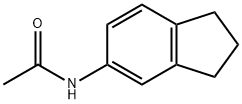
59856-06-3
44 suppliers
$60.00/1g

158205-20-0
5 suppliers
inquiry

158205-18-6
16 suppliers
inquiry

158205-20-0
5 suppliers
inquiry