
6-BroMo-2-chloro-3-Methylquinoxaline synthesis
- Product Name:6-BroMo-2-chloro-3-Methylquinoxaline
- CAS Number:98416-72-9
- Molecular formula:C9H6BrClN2
- Molecular Weight:257.51
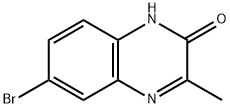
98416-69-4
2 suppliers
inquiry

98416-72-9
19 suppliers
inquiry
Yield:98416-72-9 6.67 g
Reaction Conditions:
Stage #1: 6-bromo-3-methyl-1H-quinoxalin-2-onewith P,P-dichlorophenylphosphine oxide at 150; for 3 h;
Stage #2: with ammonia in water; pH=7;
Steps:
INTERMEDIATE: (6-Bromo-3-methyl-quinoxalin-2-yl)-hydrazine (IIo). 4-Bromo-2-fluoro-l- nitro-benzene (40 g), racemic alanine (16.2 g), and K2CO3 (30 g) were refluxed overnight in a mixture of in ethanol (200 mL) and water (200 mL). After cooling to ambient temperature, the mixture was diluted with water, and acidified with 1M aq HCl. The precipitated solid was collected and dried to afford 2-(5-bromo-2-nitro-phenylamino)-propionic acid (41 g). A larger portion of this material prepared in a similar manner (60 g) was dissolved in methanol (250 mL) and treated with SOCI2 (30 mL, added drop-wise). The reaction mixture was stirred at ambient temperature overnight. The volatiles were removed in vacuo. The residue was partitioned between EtOAc and aq NaHC03. The organic layer was dried over MgSO i, filtered, and concentrated in vacuo to afford (5- bromo-2-nitro-phenylamino)-propionic acid methyl ester (60 g). This material was dissolved in AcOH (400 mL), iron powder (55 g) was added, and the mixture was refluxed for 4h. After cooling to ambient temperature, the solid was filtered off and the filtrate was concentrated in vacuo. The residue was partitioned between EtOAc and sat. aq NaHC03. The organic layer was dried over Na2S04, filtered, and concentrated in vacuo. The residue was purified by chromatography on silica (eluent: heptanes→ EtOAc) to afford 6-bromo-3-methyl-3,4-dihydro-lH-quinoxalin-2-one (47.7 g) as a pale yellow solid. A portion of this material (10 g) was dissolved in THF (150 mL), and the solution was cooled on an ice/water bath. Mn02 (19.3 g) was added. The resulting mixture was stirred at ambient temperature overnight. EtOAc (100 mL) was added to the mixture. The solid was filtered off. The filtrate was concentrated in vacuo to afford 6-bromo-3-methyl-lH-quinoxalin-2-one (8.8 g). A larger portion of this material prepared in a similar manner (10 g) was stirred in PI1POCI2 (80 mL) at 150°C for 3h. After cooling to ambient temperature, water was added and pH was adjusted to 7 with aqueous ammonia. The precipitated solid was filtered off, washed with water and dried to afford 6-bromo-2-chloro-3-methyl-quinoxaline (6.67 g). A larger portion of this material prepared in a similar manner (14 g) was dissolved in ethanol (250 mL) and hydrazine hydrate (180 mL) was added. The mixture was refluxed for 3h, cooled to ambient temperature, and most of the volatiles were removed in vacuo. The residue was diluted with water, and solid was filtered off, washed with water, and dried to afford IIo (11.2 g) as a yellow solid sufficiently pure for the next step.
References:
WO2013/34755,2013,A1 Location in patent:Page/Page column 25
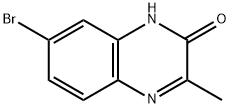
103095-19-8
10 suppliers
inquiry

98416-69-4
2 suppliers
inquiry
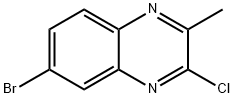
1392413-56-7
16 suppliers
inquiry

98416-72-9
19 suppliers
inquiry
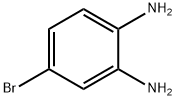
1575-37-7
368 suppliers
$10.00/1g

98416-72-9
19 suppliers
inquiry
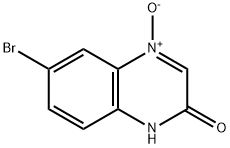
98416-75-2
0 suppliers
inquiry

98416-72-9
19 suppliers
inquiry
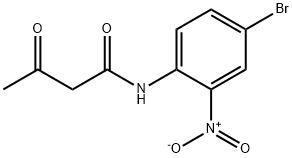
96089-46-2
0 suppliers
inquiry

98416-72-9
19 suppliers
inquiry