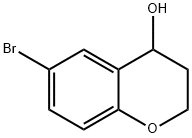
6-Bromo-3,4-Dihydro-2H-1-Benzopyran-4-Ol synthesis
- Product Name:6-Bromo-3,4-Dihydro-2H-1-Benzopyran-4-Ol
- CAS Number:18385-77-8
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07
49660-57-3
215 suppliers
$7.00/250mg

18385-77-8
27 suppliers
$155.00/50mg
Yield: 100%
Reaction Conditions:
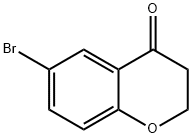
Stage #1:6-bromochroman-4-one with sodium tetrahydroborate in methanol;dichloromethane at 0 - 20; for 2 h;
Stage #2: with methanol;water in dichloromethane;ethyl acetate
Steps:
70.1
Step 1In a manner similar to that described in the literature (J. Med. Chem. 2205, 48, 1796), a solution of 6-bromo-chroman-4-one (10 g) in 1 :1 MeOH:DCM (100 ml_) was cooled an ice bath, treated with NaBH4 (1.63 g), and then stirred at RT. After 2h, the reaction was then worked up with water and EtOAc to provide compound 7OA in nearly quantitative yield. The product was used in the next step without chromatographic purification.
References:
SCHERING CORPORATION WO2008/100459, 2008, A1 Location in patent:Page/Page column 92

3875-78-3
99 suppliers
$22.00/100mg

18385-77-8
27 suppliers
$155.00/50mg