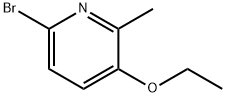
6-Bromo-3-ethoxy-2-methylpyridine synthesis
- Product Name:6-Bromo-3-ethoxy-2-methylpyridine
- CAS Number:864177-93-5
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08

75-03-6
361 suppliers
$11.00/5g
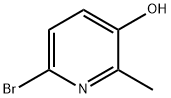
118399-86-3
95 suppliers
$43.00/100mg

864177-93-5
8 suppliers
inquiry
Yield:864177-93-5 72.7%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 0 - 45; for 20 h;
Steps:
27
Preparation 27; 6-Bromo-3-ethoxv-2-methvl-pYridine; The product of preparation 26 (3.83, 20. 3mmol) and potassium carbonate (5.6g, 40. 6mmol), were dissolved in N, N-dimethylformamide (40mL), and cooled to 0C. lodoethane (2mL, 24. 4mmol) was added dropwise and the solution was stirred for 12 hours. The mixture was then treated with additional iodoethane (0.8mL) and stirred at 45C for a further 8 hours. The solvent was evaporated under reduced pressure and azeotroped with toluene. The residue was taken up in dichloromethane and washed with water, sodium hydrogen carbonate solution and brine. The organic layer was dried over magnesium sulfate, concentrated in vacuo and the residual brown liquid was passed through a silica pad to yield the title compound, 4.4g, (72. 7%).'H NMR (CDC13, 400MHz) 5 : 1.25 (t, 3H), 2.25 (s, 3H), 4.00 (q, 2H), 6.95 (d, 1 H), 7.20 (d, 1 H). MS APCI+ m/z 218 [MH] +
References:
WO2005/82866,2005,A2 Location in patent:Page/Page column 66-67