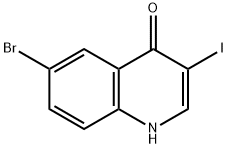
6-BroMo-3-iodo-1H-quinolin-4-one synthesis
- Product Name:6-BroMo-3-iodo-1H-quinolin-4-one
- CAS Number:1320361-71-4
- Molecular formula:C9H5BrINO
- Molecular Weight:349.95
332366-57-1
45 suppliers
$16.00/250mg

1320361-71-4
21 suppliers
$65.00/50mg
Yield:1320361-71-4 93%
Reaction Conditions:
with N-iodo-succinimide in N,N-dimethyl-formamide at 20; for 0.05 h;
Steps:
6-Bromo-3-iodoquinolin-4(1H)-one (2).
N-iodosuccinimide (3 g, 13.4 mmol) was added to a solution of 3 (2 g, 8.92 mmol) in dry DMF (27 mL) maintained under stirring at room temperature. After 3 min, the mixture was diluted with water and the precipitate which formed was filtered and washed with water and diethyl ether to afford 2 (2.9 g, 93%) as a withe solid. Rf 0.72 (CH2Cl2/MeOH, 9/1); mp >290 °C; 1H NMR (200 MHz, (CD3)2SO): δ 12.27 (s, 1H), 8.47 (s, 1H), 8.10 (d, J = 2.4 Hz, 1 H), 7.79 (dd, Jortho = 9.0 Hz, Jmeta = 2.4 Hz, 1H), 7.47 (d, J = 8.9 Hz, 1H); 13C NMR (100 MHz, (CD3)2SO) δ 172.4, 145.6, 138.9, 135.1, 128.0, 124.1, 121.6, 117.0, 81.3; IR (Nujol): n = 1614 cm-1; MS: m/z (M+ + 1) = 350.
References:
Mugnaini, Claudia;Falciani, Chiara;De Rosa, Maria;Brizzi, Antonella;Pasquini, Serena;Corelli, Federico [Tetrahedron,2011,vol. 67,# 32,p. 5776 - 5783] Location in patent:supporting information; experimental part
![2-Propenoic acid, 3-[(4-bromophenyl)amino]-, ethyl ester](/CAS/20210111/GIF/1320361-74-7.gif)
1320361-74-7
1 suppliers
inquiry

1320361-71-4
21 suppliers
$65.00/50mg

106-40-1
470 suppliers
$12.00/5g

1320361-71-4
21 suppliers
$65.00/50mg