
6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE synthesis
- Product Name:6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE
- CAS Number:1260810-02-3
- Molecular formula:C12H8BrN3
- Molecular Weight:274.12
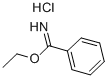
5333-86-8
134 suppliers
$21.21/5gm:
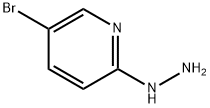
77992-44-0
216 suppliers
$8.00/1g
![6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB6133652.gif)
1260810-02-3
8 suppliers
$2165.44/10g
Yield:1260810-02-3 89%
Reaction Conditions:
with sodium acetate;acetic acid in ethanol at 70; for 24 h;
Steps:
6-Bromo-3-phenyl-[1,2,4]triazolo[4,3-a]pyridine (1e)
To a mixture of ethyl benzimidate hydrochloride(5b), 1.00 g, 5.22 mmol, 1 equiv.),5-bromo-2-hydrazinopyridine (4d), 982mg, 5.22 mmol, 1.00 equiv.) and anhydrous sodium acetate (433 mg, 5.22 mmol,1.00 equiv) was added absolute ethanol (SDA 3C, 5.00 mL) followed by acetic acid (150 μL, 2.61 mmol, 0.50 equiv).The heterogeneous mixture was heated to 70 °C for 24 h. The slurry was cooled to room temperature and concentrated in vacuo to afford a brown solid which was partitioned between dichloromethane (50 mL) and saturated aqueous sodium bicarbonate (25 mL). The layers were separated and the aqueous layer was extracted with dichloromethane (25 mL). The combined organic extracted were dried over sodium sulfate, filtered and concentrated in vacuo to afford a brown solid. The solid was purified by flash column chromatography over silica gel (0 → 10 % methanol in dichloromethane gradient) to afford (1e) as a pale orange solid (1.27 g, 89 %). A duplicate experiment afforded 1.22 g (85 %) of product.1H NMR (500 MHz, CDCl3, 23°C): δ 8.36 (m, 1H), 7.75-7.77 (m, 2H), 7.67 (dd, J = 9.2, 0.8 Hz, 1H), 7.49-7.56 (m, 3H), 7.28 (dd, J = 9.7, 1.8 Hz, 1H).13C NMR (125.8 MHz, CDCl3, 23°C): δ 148.9, 146.5, 130.6, 130.3, 129.3, 128.0, 125.9, 122.4, 117.2, 109.3.FTIR(thin film) (cm-1):3085(w), 1627 (w), 1496 (m), 1049 (w), 793 (w).HRMS(ESI) (m/z):Calc’d for C12H9BrN3[M+H]+: 273.9974,Found:273.9966.Melting Point: 174-175°C.
References:
Schmidt, Michael A.;Qian, Xinhua [Tetrahedron Letters,2013,vol. 54,# 42,p. 5721 - 5726] Location in patent:supporting information

77992-44-0
216 suppliers
$8.00/1g
611-73-4
289 suppliers
$36.96/5g
![6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB6133652.gif)
1260810-02-3
8 suppliers
$2165.44/10g
626-55-1
541 suppliers
$5.00/5/ G

1666-17-7
68 suppliers
$21.00/1g
![6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB6133652.gif)
1260810-02-3
8 suppliers
$2165.44/10g

77992-44-0
216 suppliers
$8.00/1g
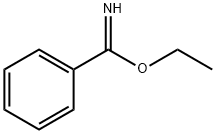
825-60-5
9 suppliers
inquiry
![6-BROMO-3-PHENYL-[1,2,4]TRIAZOLO[4,3-A]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB6133652.gif)
1260810-02-3
8 suppliers
$2165.44/10g