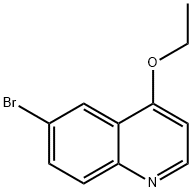
6-Bromo-4-ethoxyquinoline synthesis
- Product Name:6-Bromo-4-ethoxyquinoline
- CAS Number:879323-77-0
- Molecular formula:C11H10BrNO
- Molecular Weight:252.11

65340-70-7
262 suppliers
$7.00/250mg
141-52-6
460 suppliers
$10.00/5g

879323-77-0
5 suppliers
inquiry
Yield:879323-77-0 90.2%
Reaction Conditions:
in ethanol at 20 - 120; for 15 h;
Steps:
3.a
To a solution of 6-bromo-4-chloroquinoline (17 g, 70.10 mmol) in ethanol (400 mL) was added sodium ethoxide (23.85 g, 350.5 mmol) at room temperature. Then, the reaction mixture was heated to 120° C. for 15 h in a sealed reaction flask. After cooling to room temperature, the ethanol was removed under the vacuum and the residue was diluted with water. The aqueous suspension was neutralized with 3.0N hydrochloric acid until the precipitate formed and later it was diluted with saturated sodium bicarbonate solution. Then, the solids were collected by filtration and washed with water. After drying in air, 15.94 g (90.2% yield) of 6-bromo-4-ethoxy-quinoline was isolated as a white solid which can be crystallized from acetonitrile: EI-HRMS m/e calcd for C11H10BrNO (M+) 250.9946, found 250.9946.
References:
US2006/63805,2006,A1 Location in patent:Page/Page column 11-12

13720-91-7
13 suppliers
inquiry

879323-77-0
5 suppliers
inquiry