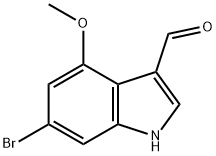
6-bromo-4-methoxy-1H-indole-3-carbaldehyde synthesis
- Product Name:6-bromo-4-methoxy-1H-indole-3-carbaldehyde
- CAS Number:1202766-19-5
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
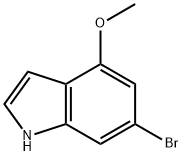
393553-57-6
99 suppliers
$256.00/5g

149409-22-3
3 suppliers
inquiry

1202766-19-5
28 suppliers
inquiry
Yield:1202766-19-5 61.9%
Reaction Conditions:
Stage #1: 6-bromo-4-methoxyindole;Vilsmeier reagent in N,N-dimethyl-formamide at 20; for 24 h;
Stage #2: with sodium hydroxide in water;N,N-dimethyl-formamide; for 1 h;Reflux;
Steps:
To a solution of 6-bromo-4-methoxy-lH-indole A.85 (2.0 g, 8.85 mmol) in DMF (9 mL) was added (chloromethylene)dimethylamrnonium chloride (1.699 g, 13.27 mmol) and the mixture was stirred at room temperature for 24 hours. To the mixture was added water (50 mL) and 10 N aqueous NaOH (10 mL) and the mixture was heated at reflux for 1 hour. The mixture was cooled to 0 0C. The resulting precipitate was collected by filtration to give 6-bromo-4- methoxy-lH-indole-3-carbaldehyde A.140 (1.392 g, 61.9% yield) as a brown solid: 1H NMR (400 MHz, DMS0-d6) δ ppm 12.26 (1 H, s), 10.26 (1 H, s), 8.05 (1 H, s), 7.30 (1 H, d, J=I .5 Hz), 6.89 (1 H, d, J=I .1 Hz), 3.95 (3 H, s); Mass Spectrum (ESI) m/e = 253.9 [M+l (79Br)] and 255.9 [M+l (81Br)].
References:
WO2009/158011,2009,A1 Location in patent:Page/Page column 80-81
43192-33-2
161 suppliers
$15.00/1g

1202766-19-5
28 suppliers
inquiry