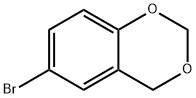
6-BROMO-4H-1,3-BENZODIOXINE synthesis
- Product Name:6-BROMO-4H-1,3-BENZODIOXINE
- CAS Number:90050-61-6
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
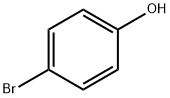
106-41-2
498 suppliers
$13.00/5g

90050-61-6
7 suppliers
inquiry
Yield:90050-61-6 75%
Reaction Conditions:
with sodium hydroxide;sulfuric acid;acetic acid;paraformaldehyde in water;toluene;
Steps:
1 6-Bromo-4H-1,3-benzodioxin
6-Bromo-4H-1,3-benzodioxin This compound is pdrepared as described in 1.b. hereinabove, starting from the following quantities of reactants: 3.8 liters of acetic acid, 654 ml of concentrated sulfuric acid, 1 kg (5.78 moles) of 4-bromophenol and 870 g (28.9 moles) of paraformaldehyde. The duration of the reaction is 120 hours at 0° C. After neutralization of the reaction mixture with a solution containing 1350 g of sodium hydroxide dissolved in 13 liters of water, the precipitate obtained is filtered off and then dissolved in 7 liters of toluene. The organic phase is dried by azeotropic distillation, filtered hot to remove formaldehyde polymers and then evaporated under reduced pressure. The residue is distilled under reduced pressure. 933 g of 6-bromo-4H-1,3-benzodioxin are obtained. Yield: 75% of theory; B.P.: 80°-90° C./0.13 mbar. The product crystallizes when stirred in a mixture of diisopropyl ether/hexane; M.P.: 43°-47° C. NMR (CDCl3): delta 4.88 (2H, s, Ar--CH2), 5.24 (2H, s, --OCH2 O--), 6.39 (1H, d, J=8.7 Hz, ArH), 7.13 (1H, m, ArH), 7.31 (1H, dd, J=8.7 and 2.4 Hz, ArH).
References:
US4814343,1989,A

254-27-3
3 suppliers
inquiry

90050-61-6
7 suppliers
inquiry