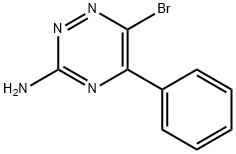
6-BROMO-5-PHENYL-1,2,4-TRIAZIN-3-AMINE synthesis
- Product Name:6-BROMO-5-PHENYL-1,2,4-TRIAZIN-3-AMINE
- CAS Number:1321517-51-4
- Molecular formula:C9H7BrN4
- Molecular Weight:251.08
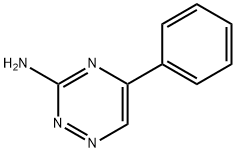
942-60-9
12 suppliers
inquiry

1321517-51-4
8 suppliers
inquiry
Yield:1321517-51-4 64%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at -25;
Steps:
3.i
A solution of intermediate C, a 5-aryl-1 ,2,4-triazin-3-amine derivative (8.70 mmol) in DMF (15 ml_) is cooled to -25 °C and treated with a solution of N-chlorosuccinimide or N- bromosuccinimide (26.6 mmol) in DMF (10 ml_) by drop wise addition. The reaction is stirred overnight and monitored by TLC (methanol/DCM, 1 :9). After completion of the reaction, the mixture is poured into saturated bicarbonate solution (50 ml_) and extracted with diethyl ether (25 χ 3ml_). The organic phases are combined, dried over Na2SC>4 and concentrated in vacuo. The crude compound is purified by gradient flash chromatography, eluting with mixtures of ethyl acetate in hexane (e.g. 10% ethyl acetate in hexane) to afford the target compound, intermediate D.Method 2A solution of intermediate C, a 5-aryl-1 ,2,4-triazin-3-amine derivative (8.70 mmol) in DMF (15 ml_) is cooled to -25 °C and treated with a solution of N-chlorosuccinimide or N- bromosuccinimide (26.6 mmol) in DMF (10 ml_) by drop wise addition. The reaction is stirred at room temperature and monitored by TLC or LCMS. After completion of the reaction, the mixture is poured into saturated bicarbonate solution (50 ml_) and extracted with an organic solvent such as diethyl ether or ethyl acetate. The organic phases are combined, dried over NaaSC and concentrated in vacuo. The crude compound is purified by gradient flash chromatography, eluting with mixtures of ethyl acetate in hexane, or methanol in DCM, to afford the target compound, intermediate D.(i) 6-Bromo-5-phenyl-1 ,2,4-triazin-3-amine6-Bromo-5-phenyl-1 ,2,4-triazin-3-amine (1.40 g, 64%) was prepared from 5-phenyl-1 ,2,4- triazin-3-amine (1.50 g, 8.70 mmol) and N-bromosuccinimide (4.50 g, 26.6 mmol) according to the general procedure of Preparation 3.Mass spectroscopy: (ESI +ve) 251.9 [M+H]+ 1H NMR: (400 MHz, CDCI3) δ: 5.49 (s, 2H), 7.49-7.58 (m, 3H), 7.82-7.85 (m, 2H).
References:
WO2011/95625,2011,A1 Location in patent:Page/Page column 103-104
28735-27-5
20 suppliers
inquiry

1321517-51-4
8 suppliers
inquiry

83413-00-7
0 suppliers
inquiry

1321517-51-4
8 suppliers
inquiry