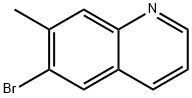
6-bromo-7-methylquinoline synthesis
- Product Name:6-bromo-7-methylquinoline
- CAS Number:122759-89-1
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
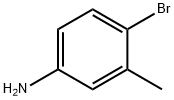
6933-10-4
183 suppliers
$7.00/5g
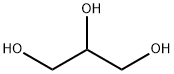
56-81-5
1636 suppliers
$5.00/25g

122759-89-1
53 suppliers
$30.00/250mg
Yield: 31%
Reaction Conditions:
Stage #1:amino-5-bromo-2-toluene;glycerol with iron(III) sulfate;sulfuric acid in nitrobenzene for 3 h;Heating / reflux;
Stage #2: with water;sodium hydrogencarbonate in nitrobenzene; pH=7 - 8
Steps:
1.4
Intermediate 4: 6-Bromo-7-methyl-quinoline; [0304] A mixture of 4-bromo-3-methylaniline (20 g, 107.5 mmol), ferric sulfate (6.6 g, 43.4 mmol), glycerol (40.8 g, 440 mmol), nitrobenzene (8.12 g, 66 mmol), and concentrated EPO
References:
SGX PHARMACEUTICALS, INC. WO2008/51808, 2008, A2 Location in patent:Page/Page column 79-80