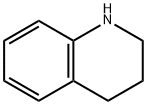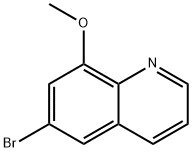
6-broMo-8-Methoxyquinoline synthesis
- Product Name:6-broMo-8-Methoxyquinoline
- CAS Number:103028-32-6
- Molecular formula:C10H8BrNO
- Molecular Weight:238.08
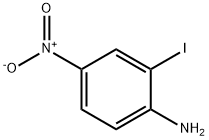
6293-83-0
154 suppliers
$6.00/250mg
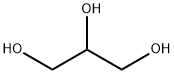
56-81-5
1634 suppliers
$5.00/25g

103028-32-6
35 suppliers
inquiry
Yield:103028-32-6 100%
Reaction Conditions:
with sulfuric acid;sodium 3-nitrobenzenesulfonate in water at 0 - 120; for 24.5 h;Inert atmosphere;
Steps:
Intermediate 49: 6-bromo-8-methoxyquinoline
4-Bromo-2-methoxyaniline (50.0 g, 247 mmol) was added to a mixture of sodium 3- nitrobenzenesulfonate (86.8 g, 385 mmol) and propane-1 ,2,3-triol (108 g, 1 .18 mol) in cone. H2SO4 (97 ml.) and water (75.7 ml.) at 0 °C over a period of 30 min under nitrogen. After stirring at 120 °C for 24 h and then cooling to RT, the reaction mixture was quenched slowly with 2M NaOH (1 L). The aq. solution was extracted with EtOAc (3 x 500 ml_). The combined organic phases were dried over Na2S04, filtered and concentrated to afford 6-bromo-8- methoxyquinoline (Intermediate 49, 60.0 g, 100%); M/Z (ES+), [M+H]+= 237.1H NMR (300 MHz, DMSO-ofe) δ 3.99 (s, 3H), 7.30 (d, 1 H), 7.58-7.60 (m, 1 H), 7.79 (s, 1 H), 8.28 (dd, 1 H), 8.86 (dd, 1 H).
References:
WO2018/178226,2018,A1 Location in patent:Page/Page column 77
3054-95-3
198 suppliers
$28.00/25g

6293-83-0
154 suppliers
$6.00/250mg

103028-32-6
35 suppliers
inquiry

67-56-1
738 suppliers
$9.00/25ml
190843-73-3
13 suppliers
inquiry

50488-36-3
29 suppliers
inquiry

103028-32-6
35 suppliers
inquiry
90-04-0
375 suppliers
$5.00/5G

103028-32-6
35 suppliers
inquiry