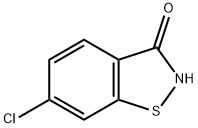
6-CHLORO-1,2-BENZISOTHIAZOL-3(2H)-ONE synthesis
- Product Name:6-CHLORO-1,2-BENZISOTHIAZOL-3(2H)-ONE
- CAS Number:70-10-0
- Molecular formula:C7H4ClNOS
- Molecular Weight:185.6308
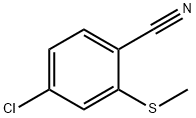
176107-21-4
2 suppliers
inquiry

70-10-0
46 suppliers
$105.00/10mg
Yield:70-10-0 97%
Reaction Conditions:
Stage #1: 4-chloro-2-(methylthio)benzonitrilewith hydrogenchloride;chlorine in water;chlorobenzene at 45 - 70; for 5 h;
Stage #2: with water;sodium hydroxide in chlorobenzene at 65 - 70;
Steps:
5 Example 5
Example 5
4-Chloro-2-(methylthio)benzonitrile (36.7 g, 0.2 mol), monochlorobenzene (50.0 g), and 35% by weight hydrochloric acid (1.1 g, water: 0.04 mol) were placed in a 500-ml four-necked flask equipped with a stirrer, a thermometer, and a condenser to give a mixture.
Chlorine (15.6 g, 0.22 mol) was blown into the mixture at 45 to 50° C over a period of 2 hours under stirring and 35% by weight hydrochloric acid (5.5 g, water: 0.2 mol) was added dropwise thereto over a period of 2 hours at the same time with blowing the chlorine.
After completion of blowing chlorine and the dropwise addition of water, the mixture was further heated to 65 to 70°C and allowed to react for 1 hour.
After completion of the reaction, a 20% by weight aqueous solution (41.0 g) of sodium hydroxide was added thereto at the same temperature and the mixture was cooled to room temperature.
The precipitated crystal was collected by filtration, washed with monochlorobenzene, and dried to obtain 6-chloro-1,2-benzisothiazol-3-one (36.0 g, 0.194 mol).
The yield of the target product relative to 4-chloro-2-(methylthio)benzonitrile was 97%.
References:
EP2687519,2014,A1 Location in patent:Paragraph 0047; 0048
131002-01-2
15 suppliers
$72.00/1g

70-10-0
46 suppliers
$105.00/10mg
942319-20-2
4 suppliers
inquiry

70-10-0
46 suppliers
$105.00/10mg

19993-68-1
0 suppliers
inquiry

70-10-0
46 suppliers
$105.00/10mg
19993-69-2
0 suppliers
inquiry

70-10-0
46 suppliers
$105.00/10mg