
6-Chloro-1H-quinolin-4-one synthesis
- Product Name:6-Chloro-1H-quinolin-4-one
- CAS Number:21921-70-0
- Molecular formula:C9H6ClNO
- Molecular Weight:179.6
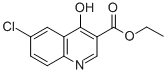
70271-77-1
65 suppliers
$18.00/250mg

21921-70-0
18 suppliers
$45.00/250mg
Yield:21921-70-0 97 %
Reaction Conditions:
Stage #1: ethyl 6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylatewith sodium hydroxideReflux;
Stage #2: with hydrogenchloride at 20;
Stage #3: in diphenylether;Reflux;
Steps:
General procedure C: decarboxylation
General procedure: According to a modified literature procedure [29], quinolonecarboxylates 3a-3d (1.00 equiv.) were suspended in 2 NNaOH solution (20 cm3/mmol). The reaction mixture washeated to reflux, up to 4 h, until full conversion was observedby TLC (CH2Cl2/MeOH, 9:1). The reaction mixture wascooled to rt and neutralized with 2 N HCl to precipitate theproduct. The product was collected by filtration, washed with200 cm3water and dried. Decarboxylation was performedin Ph2O(20 cm3/mmol). Carboxylic acid (1.00 equiv.) wassuspended and heated to reflux (250 °C) up to 2 h until fullconversion was observed by TLC (CH2Cl2/MeOH, 9:1). Thereaction mixture was cooled to rt and poured into LP to precipitatethe desired products 4a-4d which were collected byfiltration, washed several times with LP and dried.
References:
Draskovits, Markus;Catorci, Daniele;Wimmer, Laurin;Rehman, Sabah;Siebert, David Chan Bodin;Ernst, Margot;Schnürch, Michael;Mihovilovic, Marko D. [Monatshefte fur Chemie,2022]
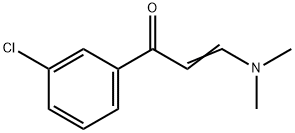
75175-79-0
10 suppliers
inquiry

21921-70-0
18 suppliers
$45.00/250mg

106-47-8
463 suppliers
$5.00/5G

21921-70-0
18 suppliers
$45.00/250mg

19056-79-2
18 suppliers
$28.60/10MG

21921-70-0
18 suppliers
$45.00/250mg
![5-[(4-Chloro-phenylaMino)-Methylene]-2,2-diMethyl-[1,3]dioxane-4,6-dione](/CAS/GIF/25063-46-1.gif)
25063-46-1
12 suppliers
$60.00/50mg

21921-70-0
18 suppliers
$45.00/250mg