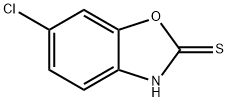
6-Chloro-2-benzoxazolethiol synthesis
- Product Name:6-Chloro-2-benzoxazolethiol
- CAS Number:22876-20-6
- Molecular formula:C7H4ClNOS
- Molecular Weight:185.63

19932-84-4
136 suppliers
$7.00/1g

22876-20-6
121 suppliers
$31.80/1gm:
Yield:22876-20-6 98.9%
Reaction Conditions:
Stage #1: 6-chlorobenzo[d]oxazol-2(3H)-onewith sodium hydroxide in water at 85; for 2 h;
Stage #2: with carbon disulfide at 30 - 135; under 2250.23 Torr; for 0.00833333 h;Temperature;Pressure;
Steps:
1-6 Example 2:
Dissolve 850.0g (5.01mol) of 6-chlorobenzoxazolone in 25% NaOH solution (1843.7g, 11.52mol), heat to 85°C, react for 2h, put the reaction solution in the A bottle of the metering pump, Weigh 389.3g CS2 (5.11mol) and place it in the B bottle of the metering pump. Turn on the temperature control system, adjust the metering pumps, and control the molar ratio of the precursor salt and carbon disulfide to 1:1.02, and enter the mixed template. The mixed template is kept at a constant temperature of 30°C, and then the two raw materials enter the microchannel reaction template simultaneously. Carry out the sulfhydryl cyclization reaction. The reaction template is thermostatted at 135°C and the residence time is 30s. The pressure of the reaction system is 0.3MPa by adjusting the back pressure valve. Then the template is cooled and the template is cooled at 10°C. After staying for 30s, it exits from the microchannel The outlet of the reactor flows into a 20% dilute hydrochloric acid solution (1921.2g). Stop flowing into the acidification kettle when the system pH=6. Stir the material with pH=6 at 25 for 30min, centrifuge and dry to obtain 2-mercapto-6-chloride Benzoxazole 920.3g, content (99.2%), yield 98.9% (calculated based on 6-chlorobenzoxazolone).
References:
CN111423392,2020,A Location in patent:Paragraph 0007; 0017-0022

75-15-0
236 suppliers
$40.00/100g

28443-50-7
230 suppliers
$21.00/5g

22876-20-6
121 suppliers
$31.80/1gm:

137-26-8
301 suppliers
$10.00/25g

28443-50-7
230 suppliers
$21.00/5g

22876-20-6
121 suppliers
$31.80/1gm:
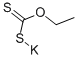
140-89-6
303 suppliers
$12.00/5g

28443-50-7
230 suppliers
$21.00/5g

22876-20-6
121 suppliers
$31.80/1gm:

75-15-0
236 suppliers
$40.00/100g

19932-84-4
136 suppliers
$7.00/1g

22876-20-6
121 suppliers
$31.80/1gm: