
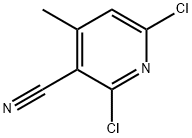
6-Chloro-2-Methoxy-4-Methylnicotinonitrile synthesis
- Product Name:6-Chloro-2-Methoxy-4-Methylnicotinonitrile
- CAS Number:243469-65-0
- Molecular formula:C8H7ClN2O
- Molecular Weight:182.61

124-41-4
683 suppliers
$12.00/25g
875-35-4
270 suppliers
$13.00/25g

51564-29-5
6 suppliers
inquiry

243469-65-0
5 suppliers
inquiry
Yield:243469-65-0 32.3% ,51564-29-5 40.4%
Reaction Conditions:
in methanol at 20; for 16 h;
Steps:
Intermediate 114A: 6-chloro-2-methoxy-4-methylnicotinonitrile and (1445) Intermediate 114B: 2-chloro-6-methoxy-4-methylnicotinonitrile
To a stirred solution of 2,6-dichloro-4-methylnicotinonitrile (15.00 g, 80.00 mmol) in MeOH (100 mL) was added sodium methoxide (14.89 mL, 80.00 mmol) and the reaction mixture was stirred at ambient temperature for 16 h. The reaction mixture was diluted with water (300 mL) and extracted with DCM (3 x 250 mL). The combined organic layers were washed with brine (200 mL), dried over anhydrous sodium sulfate and evaporated under reduced pressure. The residue was purified by SFC [Column: Lux cellulose-2 (50 x 250 mm) 5 micron; 10% of 0.2% DEA in IPA; total flow: 150 g/min; UV: 220nm] to obtain Intermediate 114A (5.50 g, 32.30%) as an off-white solid, (retention time: 6.3 min). 1H NMR (400 MHz, DMSO-d6) G ppm 2.46 (s, 3 H), 3.99 (s, 3 H), 7.27 (s, 1 H). LCMS: (Method-I) retention time: 1.22 min, [M+1] 183.3. Intermediate 114B (6.50 g, 40.40%) as an off-white solid, (retention time: 5.8 min).1H NMR (400 MHz, DMSO-d6) G ppm 2.43 (s, 3 H), 3.91 (s, 3 H), 6.94 (s, 1 H). LCMS (Method-I): retention time 1.29 min, [M+1] 183.4
References:
WO2018/222795,2018,A1 Location in patent:Page/Page column 242

67-56-1
738 suppliers
$9.00/25ml

124-41-4
683 suppliers
$12.00/25g

875-35-4
270 suppliers
$13.00/25g

51564-29-5
6 suppliers
inquiry

243469-65-0
5 suppliers
inquiry
5444-02-0
204 suppliers
$10.00/5g

243469-65-0
5 suppliers
inquiry