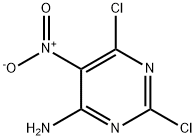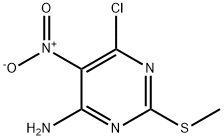
6-Chloro-2-(methylthio)-5-nitropyrimidin-4-amine synthesis
- Product Name:6-Chloro-2-(methylthio)-5-nitropyrimidin-4-amine
- CAS Number:38136-96-8
- Molecular formula:C5H5ClN4O2S
- Molecular Weight:220.64
1979-96-0
87 suppliers
$19.00/250mg

38136-96-8
19 suppliers
inquiry
Yield:38136-96-8 100%
Reaction Conditions:
with ammonia;triethylamine in tetrahydrofuran;isopropanol at 20;Cooling;
Steps:
8 Synthesis of intermediate I-2
A solution of NH3 2M in iPrOH (1 15 mL, 229.31 mmol) was added drop wise to a solution of H-2 (36.7 g, 152.87 mmol) and Et3N (23.4 mL, 168.16 mmol) in THF (360 mL) (the temperature was maintained at RT with an ice-water bath during the addition). The reaction mixture was stirred at RT for 5h. The mixture was evaporated to dryness. Water and EtOAc were added to the residue. The layers were separated and the aqueous layer was extracted with EtOAc (twice). The combined organic layers were dried over MgS04, filtered, and the solvent was removed under reduced pressure to give 34.5 g (100% yield) of intermediate I-2.
References:
WO2013/68438,2013,A1 Location in patent:Page/Page column 30
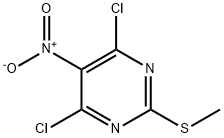
1979-98-2
234 suppliers
$14.00/5g

38136-96-8
19 suppliers
inquiry

1979-97-1
28 suppliers
inquiry

38136-96-8
19 suppliers
inquiry