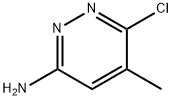
6-chloro-5-Methyl-3,6-dihydropyridazin-3-aMine synthesis
- Product Name:6-chloro-5-Methyl-3,6-dihydropyridazin-3-aMine
- CAS Number:66346-87-0
- Molecular formula:C5H6ClN3
- Molecular Weight:143.57
19064-64-3
248 suppliers
$6.00/5g

66346-87-0
120 suppliers
$9.00/100mg
Yield:66346-87-0 72.7%
Reaction Conditions:
with ammonia in ethanol;water at 100; for 48 h;
Steps:
5
EXAMPLE 5; SYNTHESIS OF IMIDAZO[1 ,2-B]PYRIDAZINE COMPOUNDS AND 7- METHYL-IMIDAZO[1 ,2-B]PYRIDAZINE COMPOUNDS [0177] Additional compounds of the invention, including illustrative compounds set forth in Table VII, were made according to the following synthetic examples. In these examples, Compound 7-12 was prepared as described above in Example 2, while compound 11 was prepared as follows:β-chloroS-methylpyridazin-S-amine 7:for 48 h.[0178] 3,6-dichloro-4-methylpyridazine 6 (1 g) was dissolved in 5 mL of ethanol and was added ammonium hydroxide (10 mL). The resulting reaction mixture was sealed in a pressure bottle and heated to 100 0C for 48 hours. The reaction mixture is cooled and the solvents were evaporated and purified by CombiFlash Companion using Hexane/DCM 40:60 solvent system (4 g normal phase RediSep Flash column with run time min at flow 18 mL/min) gave 0.640 g (72.7 %) of 7 as yellow solid).[0179] 1H-NMR (300MHz, CD3OD) 7.08 (s, 1 H), 4.72 (s, 2H), 2.27 (s, 3H), ESI-MS m/z 143.9 (M+H)+.
References:
WO2008/58126,2008,A2 Location in patent:Page/Page column 65

19064-64-3
248 suppliers
$6.00/5g

66346-87-0
120 suppliers
$9.00/100mg
64068-00-4
104 suppliers
$40.00/250mg
66530-56-1
11 suppliers
inquiry

66346-87-0
120 suppliers
$9.00/100mg