
6-chloro-7-fluoro-2,3-diphenylquinoxaline synthesis
- Product Name:6-chloro-7-fluoro-2,3-diphenylquinoxaline
- CAS Number:218456-76-9
- Molecular formula:C20H12ClFN2
- Molecular Weight:334.77
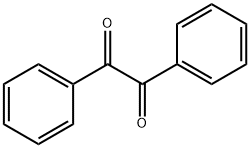
134-81-6
466 suppliers
$9.00/25g
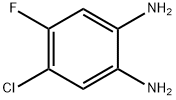
139512-70-2
117 suppliers
$9.00/250mg

218456-76-9
6 suppliers
inquiry
Yield:218456-76-9 82%
Reaction Conditions:
with acetic acid for 2.5 h;Reflux;
Steps:
8 4.5.8. Synthesis of 2,3-diphenyl-6-chloro-7-fluoroquinoxaline (7d)
Intermediate compound 5 (0.2 g, 1.24 mmol) and benzil 6d (0.26 g, 1.24 mmol) were weighed and dissolved in 20 mL glacial acetic acid.
This mixture was refluxed for 2.5hr.
The completion of reaction was monitored by TLC.
The reaction mixture was cooled and poured into crushed ice.
The precipitated product was filtered off and washed with cold water.
The product was then purified by column chromatography using hexane: ethyl acetate as mobile phase.
The pure fractions obtained were dried and concentrated to obtain white solids of product 7d. (yield 82%, m.p. 110-112 °C).
1H NMR (400 MHz, CDCl3), δ ppm, 7.3381-7.2488 (m, 6H, Ar-3,4,5-H at C-2 and C-3), 7.441-7.411 (m, 4H, Ar-2,6-H at C-2 and C-3), 7.8824 (d, J = 9.28, -H at C-5), 8.1930 (d, J = 7.6, -H at C-8).
13C NMR (400 MHz, CDCl3), δ ppm, 113.66, 113.87, 125.44, 125.66, 128.38, 129.18, 129.27, 129.78, 129.81, 130.38, 138.38, 138.53, 138.46, 140.50, 140.61, 153.73, 153.76, 154.39, 156.93, 159.46.
Mass (ESI), C20H12ClFN2, M+ and [M+2]+ peaks were found at 334 and 336, respectively.
References:
Patel, Saloni B.;Patel, Bhumika D.;Pannecouque, Christophe;Bhatt, Hardik G. [European Journal of Medicinal Chemistry,2016,vol. 117,p. 230 - 240]
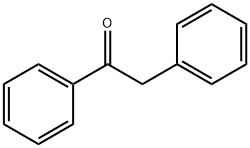
451-40-1
389 suppliers
$13.43/1gm:

139512-70-2
117 suppliers
$9.00/250mg

218456-76-9
6 suppliers
inquiry
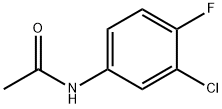
877-90-7
71 suppliers
$14.00/1g

218456-76-9
6 suppliers
inquiry
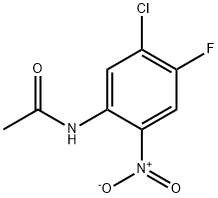
81962-58-5
26 suppliers
$10.00/250mg

218456-76-9
6 suppliers
inquiry
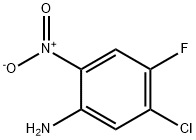
104222-34-6
122 suppliers
$9.00/1g

218456-76-9
6 suppliers
inquiry