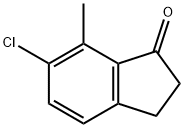
6-CHLORO-7-METHYL-1-INDANONE synthesis
- Product Name:6-CHLORO-7-METHYL-1-INDANONE
- CAS Number:628732-10-5
- Molecular formula:C10H9ClO
- Molecular Weight:180.63
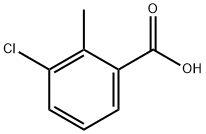
7499-08-3
177 suppliers
$8.00/10g
74-85-1
101 suppliers
$79.00/11l

628732-10-5
30 suppliers
$130.00/100mg
Yield:628732-10-5 72%
Reaction Conditions:
Stage #1: 3-chloro-2-methylbenzoic acidwith thionyl chloride in benzene;Heating / reflux;
Stage #2: ethenewith aluminum (III) chloride in 1,1-dichloroethane at 10 - 20;
Stage #3: with sulfuric acid in water at 85; for 1 h;
Steps:
NINETEEN-4 Example NINETEEN-4 (Compound 152)
Example NINETEEN-4 (Compound 152) Thionyl chloride (10.0 [ML,] 1.5 eq) and 3-chloro-2-methyl-benzoic acid (commercially available from Aldrich) (15.6 g, 91.4 mmol) in benzene was refluxed until no more gas evolution was observed. After cooling to rt the mixture was concentrated. The concentrate was diluted with dichloroethane and added to a solution [OF OF ALCL3] (12.2 g, 1.0 [EQ)] in dichloroethane at [10-20 °C. ETHYLENE WAS] bubbled through the mixture for 4 h. and the mixture was stirred overnight. The mixture was quenched with 4 N [HC1.] The resulting layers were separated and the aqueous layer was extracted with [ET20] (3 x 250 mL). The combined organic extract was washed with [H20] (3 x 150 mL), saturated [NAHC03] (3 x 150 mL), brine (1 [X] 150 mL), dried over MgS04 and concentrated. Concentrated sulfuric acid was added and the mixture was stirred at [85 °C FOR] 1 h. After cooling to rt, the reaction mixture was quenched with ice-water. The mixture was extracted with Et2O (3 x 250 mL) and the combined organic extracts were washed with [H20] (3 x 200 mL), saturated [NAHC03] (3 x 200 mL), brine (1 x 100 mL), dried over MgS04 and concentrated. Pure 6-chloro-7- methyl-l-indanone (11.9 g, 72%) was obtained after column chromatography using 20 % EtOAc: hexane as eluant. Use [OF 6-CHLORO-7-METHYL-1-INDANONE] in Method NINETEEN produced 4- (5-chloro-4-methyl-indan-2-yl)-1, 3-dihydro-imidazole-2- thione (Compound 152). [1H] NMR (300 MHz, [MEOH-D4)] 8 7.16 (d, J= 8.5 Hz, 1H), 7.02 (d, J= 8.5 Hz, [1H),] 6.62 (s, 1H), 3.59-3. 52 (m, 1H), 3.31-3. 24 (m, 2H), 3.00-2. 92 (m, 2H), 2.30 (s, 3H).
References:
WO2003/99795,2003,A1 Location in patent:Page 112

7499-08-3
177 suppliers
$8.00/10g

50-00-0
860 suppliers
$10.00/25g
108-59-8
599 suppliers
$5.00/25g

628732-10-5
30 suppliers
$130.00/100mg