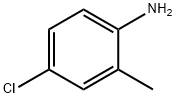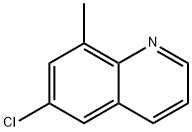
6-chloro-8-methylquinoline synthesis
- Product Name:6-chloro-8-methylquinoline
- CAS Number:19655-50-6
- Molecular formula:C10H8ClN
- Molecular Weight:177.63
Yield:19655-50-6 80%
Reaction Conditions:
with sulfuric acid in nitrobenzene at 25 - 140; for 6 h;
Steps:
1.i Preparation of 6-Chloro-8-methyl quinoline
To a stirred solution of 4-ch loro-2-methyl anil ine ( 1 00 grams, 0.706 mole) and glycerol (260 grams, 2.82 mole) in n itrobenzene (200 mL) was added concentrated sulfuric acid (200 mL) drop wise at room temperature (RT). Then reaction mass was slowly heated to 140 °C, at which temperature a vigorous reaction was observed. Mass temperature went up to reflux temperature (~ 200 °C) due to sudden exotherm. The reaction mass was further stirred for 6 hours at 140 °C, while monitoring the progress of the reaction by thin layer chromatography (TLC). After completion of reaction, the mass was cooled to RT and stirred over night. Reaction mass was quenched onto chilled water (5 L) and the pH was adjusted to ~ 9 using 40% aqueous sodium hydroxide solution. Ethyl acetate (3 L) was added to the reaction mass and stirred further for 30 minutes. The resulting solution was filtered through celite bed. Organic layer was separated and the aqueous phase was extracted with ethyl acetate (5 x 2L). The combined organic layer ( 13 L) was washed with water (3 L) and brine solution (3 L). The organic phase was dried over sodium sulfate and concentrated under vacuum to obtain the crude residue, which was further purified by flash chromatography using ethyl acetatem-hexane (5: 95) to afford the title compound. Yield: 100.8 grams (80 %). - NMR (δ ppm): 2.82 (3H, s), 7.43 - 7.46 ( 1 H. m), 7.55 ( I H, s), 7.67 - 7.68 ( 1 H, d, J = 1 .96 Hz), 8.06 - 8.08 ( I H, dd, J = 8.28, 1 .56 Hz), 8.94 - 8.95 ( 1 H, m); Mass (m/z): 178.2 (M+H)+ , 180.2 (M+H)+
References:
WO2014/147636,2014,A1 Location in patent:Page/Page column 21; 22
5202-86-8
55 suppliers
$28.60/50MG
56-81-5
1635 suppliers
$5.00/25g

19655-50-6
45 suppliers
$131.00/1g
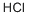
7647-01-0
11 suppliers
$10.00/10g
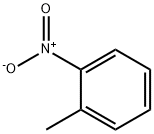
88-72-2
249 suppliers
$15.00/5g

56-81-5
1635 suppliers
$5.00/25g

19655-50-6
45 suppliers
$131.00/1g