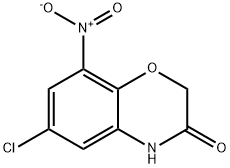
6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE synthesis
- Product Name:6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE
- CAS Number:870064-73-6
- Molecular formula:C8H5ClN2O4
- Molecular Weight:228.59

6358-08-3
134 suppliers
$10.00/1g

79-04-9
351 suppliers
$12.00/5g
![6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/870064-73-6.gif)
870064-73-6
27 suppliers
$85.00/100mg
Yield:870064-73-6 72%
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;potassium carbonate in chloroform at 0 - 55;
Steps:
27 Synthesis of 6-chloro-8-nitro-2H-benzo[b][1.4]oxazin-3 (4H)-one
Example 27 Preparation of 3-Oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-8-sulfonyl Chloride Synthesis of 6-chloro-8-nitro-2H-benzo[b][1.4]oxazin-3 (4H)-one Into a 5000 mL 3-necked round-bottom flask, was placed a solution of 2-amino-4-chloro-6-nitrophenol (40 g, 212.09 mmol, 1.00 equiv) in CHCl3 (2500 mL). To this was added N-benzyl-N-chloro-N,N-diethylethanamine (TEBA, 48 g, 210.53 mmol, 1.00 equiv). To the mixture was added K2CO3 (88 g, 637.68 mmol, 3.00 equiv). This was followed by the addition of a solution of 2-chloroacetyl chloride (28.8 g, 254.87 mmol, 1.20 equiv) in CHCl3 (500 mL), which was added dropwise with stirring, while cooling to a temperature of 0-5° C. The resulting solution was allowed to react, with stirring, for 1 h while the temperature was maintained at 0-5° C. in a bath of ice/salt. The reaction progress was monitored by TLC (EtOAc/PE=1:5). The resulting solution was allowed to react, with stirring, overnight while the temperature was maintained at 55° C. in a bath of oil. The reaction progress was monitored by TLC (EtOAc/PE=1:5). A filtration was performed. The filtrate was concentrated by evaporation under vacuum using a rotary evaporator. The resulting solution was diluted with 500 mL of H2O. A filtration was performed. The final product was purified by recrystallization from EtOH. This resulted in 34.7 g (72%) of 6-chloro-8-nitro-2H-benzo[b][1,4]oxazin-3(4R)-one as a brown solid.
References:
US2008/318941,2008,A1 Location in patent:Page/Page column 42

6358-08-3
134 suppliers
$10.00/1g

105-36-2
346 suppliers
$5.00/5G
![6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/870064-73-6.gif)
870064-73-6
27 suppliers
$85.00/100mg
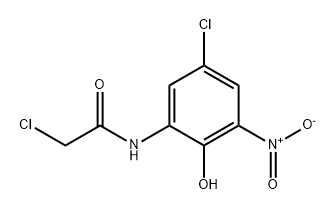
908863-01-4
0 suppliers
inquiry
![6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/870064-73-6.gif)
870064-73-6
27 suppliers
$85.00/100mg

96-35-5
316 suppliers
$5.00/5g

6358-08-3
134 suppliers
$10.00/1g
![6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/870064-73-6.gif)
870064-73-6
27 suppliers
$85.00/100mg
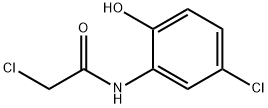
35588-38-6
9 suppliers
$60.00/100mg
![6-CHLORO-8-NITRO-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/870064-73-6.gif)
870064-73-6
27 suppliers
$85.00/100mg