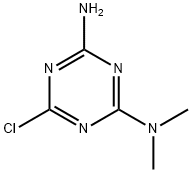
6-chloro-N,N-dimethyl-1,3,5-triazine-2,4-diamine synthesis
- Product Name:6-chloro-N,N-dimethyl-1,3,5-triazine-2,4-diamine
- CAS Number:32998-04-2
- Molecular formula:C5H8ClN5
- Molecular Weight:173.6
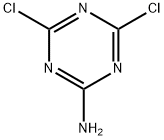
933-20-0
139 suppliers
$12.00/250mg
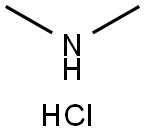
506-59-2
539 suppliers
$5.00/5G

32998-04-2
15 suppliers
$189.00/500mg
Yield:32998-04-2 150 mg
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 20; for 1 h;
Steps:
440
To a solution of 4,6-dichloro-1,3,5-triazin-2-amine 306a (300 mg, 2.72 mmol) in THF (5.0 mL) was added dimethylamine hydrochloride (220 mg, 1.00 mmol) and DIPEA (703 mg, 5.45 mmol). The reaction mixture was stirred at ambient temperature for 1 h and then quenched with water. The precipitate formed was collected by filtration, washed with water and dried to give 6-chloro-N2,N2-dimethyl-1,3,5-tnazine-2,4-diamine 306e (150 mg). The crude material was used in the next step without further purification. ESI+APCI MS m/z 174 [M + H ] + Compound 6-(4-(2-(5-chloro-2,4-dimethoxyphenyl)imidazo[l,2-a]pyridin-7-yl)piperazin-1-yl)- N2,N2-dimethyl-1, 3,5-triazine-2,4-diamine 307e was prepared in the same manner as 4-chloro-6-(4-(2-(5-chloro-2,4-dimethoxyphenyl)imidazo[1,2-a]pyridin-7-yl)piperazin-1-yl)-1,3,5-triazin-2-amine 307a, and was obtained as an off-white solid (22% yield). 1H NMR (300 MHz, DMSO-d6): δ 8.36 (d, J= 7.5 Hz, 1 H), 8.14(s, 1H), 8 ,05 (s, 1H), 6.90 - 6.88 (m, 21 1 ). 6.72 (s, 1H),6.25 (bs, 2H), 4.01 (s, 3H), 3.94 (s, 3H), 3.88 - 3.80 (m, 4H), 3.28 - 3.21 (rn, 4H), 3.02 (s, 6H). HPLC (Method 5) 99.69% (AUC), fR 12 39 min. ESI+APCI MS m/z 510 | .M + H ]
References:
WO2017/58503,2017,A1 Location in patent:Page/Page column 330; 333

933-20-0
139 suppliers
$12.00/250mg

124-40-3
503 suppliers
$18.00/100ml

32998-04-2
15 suppliers
$189.00/500mg

529510-96-1
0 suppliers
inquiry
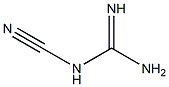
780722-26-1
2 suppliers
inquiry

32998-04-2
15 suppliers
$189.00/500mg